
A Geiger counter is an electronic instrument used for detecting and measuring ionizing radiation. It is widely used in applications such as radiation dosimetry, radiological protection, experimental physics and the nuclear industry.

Neutron activation analysis (NAA) is a nuclear process used for determining the concentrations of elements in many materials. NAA allows discrete sampling of elements as it disregards the chemical form of a sample, and focuses solely on atomic nuclei. The method is based on neutron activation and thus requires a neutron source. The sample is bombarded with neutrons, causing its constituent elements to form radioactive isotopes. The radioactive emissions and radioactive decay paths for each element have long been studied and determined. Using this information, it is possible to study spectra of the emissions of the radioactive sample, and determine the concentrations of the various elements within it. A particular advantage of this technique is that it does not destroy the sample, and thus has been used for the analysis of works of art and historical artifacts. NAA can also be used to determine the activity of a radioactive sample.
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess numbers of either neutrons or protons, giving it excess nuclear energy, and making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transferred to one of its electrons to release it as a conversion electron; or used to create and emit a new particle (alpha particle or beta particle) from the nucleus. During those processes, the radionuclide is said to undergo radioactive decay. These emissions are considered ionizing radiation because they are energetic enough to liberate an electron from another atom. The radioactive decay can produce a stable nuclide or will sometimes produce a new unstable radionuclide which may undergo further decay. Radioactive decay is a random process at the level of single atoms: it is impossible to predict when one particular atom will decay. However, for a collection of atoms of a single nuclide the decay rate, and thus the half-life (t1/2) for that collection, can be calculated from their measured decay constants. The range of the half-lives of radioactive atoms has no known limits and spans a time range of over 55 orders of magnitude.

Petrochemicals are the chemical products obtained from petroleum by refining. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as maize, palm fruit or sugar cane.

A thermoplastic, or thermosoftening plastic, is any plastic polymer material that becomes pliable or moldable at a certain elevated temperature and solidifies upon cooling.
Radiation dosimetry in the fields of health physics and radiation protection is the measurement, calculation and assessment of the ionizing radiation dose absorbed by an object, usually the human body. This applies both internally, due to ingested or inhaled radioactive substances, or externally due to irradiation by sources of radiation.

Polycarbonates (PC) are a group of thermoplastic polymers containing carbonate groups in their chemical structures. Polycarbonates used in engineering are strong, tough materials, and some grades are optically transparent. They are easily worked, molded, and thermoformed. Because of these properties, polycarbonates find many applications. Polycarbonates do not have a unique resin identification code (RIC) and are identified as "Other", 7 on the RIC list. Products made from polycarbonate can contain the precursor monomer bisphenol A (BPA).

Polyvinyl fluoride (PVF) or –(CH2CHF)n– is a polymer material mainly used in the flammability-lowering coatings of airplane interiors and photovoltaic module backsheets. It is also used in raincoats and metal sheeting. Polyvinyl fluoride is a thermoplastic fluoropolymer with a repeating vinyl fluoride unit, and it is structurally very similar to polyvinyl chloride.
The ionization chamber is the simplest type of gaseous ionisation detector, and is widely used for the detection and measurement of many types of ionizing radiation, including X-rays, gamma rays, alpha particles and beta particles. Conventionally, the term "ionization chamber" refers exclusively to those detectors which collect all the charges created by direct ionization within the gas through the application of an electric field. It uses the discrete charges created by each interaction between the incident radiation and the gas to produce an output in the form of a small direct current. This means individual ionising events cannot be measured, so the energy of different types of radiation cannot be differentiated, but it gives a very good measurement of overall ionising effect.
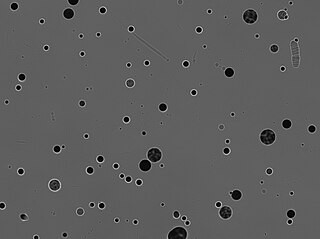
A solid-state nuclear track detector or SSNTD is a sample of a solid material exposed to nuclear radiation, etched in a corrosive chemical, and examined microscopically. When the nuclear particles pass through the material they leave trails of molecular damage, and these damaged regions are etched faster than the bulk material, generating holes called tracks.

Neutron activation is the process in which neutron radiation induces radioactivity in materials, and occurs when atomic nuclei capture free neutrons, becoming heavier and entering excited states. The excited nucleus decays immediately by emitting gamma rays, or particles such as beta particles, alpha particles, fission products, and neutrons. Thus, the process of neutron capture, even after any intermediate decay, often results in the formation of an unstable activation product. Such radioactive nuclei can exhibit half-lives ranging from small fractions of a second to many years.

A hot particle is a microscopic piece of radioactive material that can become lodged in living tissue and deliver a concentrated dose of radiation to a small area. A generally accepted theory proposes that hot particles within the body are vastly more dangerous than external emitters delivering the same dose of radiation in a diffused manner. Other researchers claim that there is little or no difference in risk between internal and external emitters, maintaining that individuals will likely continue to accumulate radiation dose from internal sources even after being removed from the original hazard and properly decontaminated, regardless of the relative danger from an internally sourced radiation dose compared to an equivalent externally sourced radiation dose.

Neutron detection is the effective detection of neutrons entering a well-positioned detector. There are two key aspects to effective neutron detection: hardware and software. Detection hardware refers to the kind of neutron detector used and to the electronics used in the detection setup. Further, the hardware setup also defines key experimental parameters, such as source-detector distance, solid angle and detector shielding. Detection software consists of analysis tools that perform tasks such as graphical analysis to measure the number and energies of neutrons striking the detector.
In health physics, whole-body counting refers to the measurement of radioactivity within the human body. The technique is primarily applicable to radioactive material that emits gamma rays. Alpha particle decays can also be detected indirectly by their coincident gamma radiation. In certain circumstances, beta emitters can be measured, but with degraded sensitivity. The instrument used is normally referred to as a whole body counter.

Radiation monitoring involves the measurement of radiation dose or radionuclide contamination for reasons related to the assessment or control of exposure to radiation or radioactive substances, and the interpretation of the results.

Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay but may also be produced in other ways. Alpha particles are named after the first letter in the Greek alphabet, α. The symbol for the alpha particle is α or α2+. Because they are identical to helium nuclei, they are also sometimes written as He2+
or 4
2He2+
indicating a helium ion with a +2 charge. Once the ion gains electrons from its environment, the alpha particle becomes a normal helium atom 4
2He.
Radioanalytical chemistry focuses on the analysis of sample for their radionuclide content. Various methods are employed to purify and identify the radioelement of interest through chemical methods and sample measurement techniques.

A radionuclide identification device is a small, lightweight, portable gamma-ray spectrometer used for the detection and identification of radioactive substances. As RIIDs are portable, they are suitable for medical and industrial applications, fieldwork, geological surveys, first-line responders in Homeland Security, and Environmental Monitoring and Radiological Mapping along with other industries that necessitate the identification of radioactive substances..
Columbia-Southern Chemical Corporation was a subsidiary of Pittsburgh Plate Glass Company. It produced heavy industrial chemicals for industry and agriculture, including: anhydrous ammonia, caustic soda, chlorine, titanium tetrachloride, and soda ash.
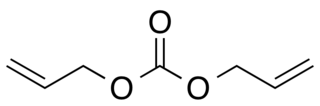
Diallyl carbonate (DAC) is a colorless liquid with a pungent odor. Its structure contains allyl groups and a functional carbonate group. The presence of double bonds in the allyl groups makes it reactive in various chemical processes. This compound plays a key role in the production of polymers, including polycarbonates and polyurethanes. Diallyl carbonate is soluble in ethanol, methanol, toluene, and chloroform. Diallyl carbonate reacts with amines, alcohols, and thiols.















