
In chemistry, a carbide usually describes a compound composed of carbon and a metal. In metallurgy, carbiding or carburizing is the process for producing carbide coatings on a metal piece.

Fluorocarbons are chemical compounds with carbon-fluorine bonds. Compounds that contain many C-F bonds often have distinctive properties, e.g., enhanced stability, volatility, and hydrophobicity. Several fluorocarbons and their derivatives are commercial polymers, refrigerants, drugs, and anesthetics.
A period 2 element is one of the chemical elements in the second row of the periodic table of the chemical elements. The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behavior of the elements as their atomic number increases; a new row is started when chemical behavior begins to repeat, creating columns of elements with similar properties.

Carbon is capable of forming many allotropes due to its valency. Well-known forms of carbon include diamond and graphite. In recent decades, many more allotropes have been discovered and researched, including ball shapes such as buckminsterfullerene and sheets such as graphene. Larger-scale structures of carbon include nanotubes, nanobuds and nanoribbons. Other unusual forms of carbon exist at very high temperatures or extreme pressures. Around 500 hypothetical 3‑periodic allotropes of carbon are known at the present time, according to the Samara Carbon Allotrope Database (SACADA).
In chemistry, an interhalogen compound is a molecule which contains two or more different halogen atoms and no atoms of elements from any other group.
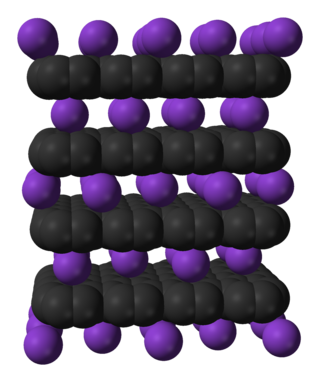
In chemistry, intercalation is the reversible inclusion or insertion of a molecule into layered materials with layered structures. Examples are found in graphite and transition metal dichalcogenides.

Manganese(III) fluoride (also known as Manganese trifluoride) is the inorganic compound with the formula MnF3. This red/purplish solid is useful for converting hydrocarbons into fluorocarbons, i.e., it is a fluorination agent. It forms a hydrate and many derivatives.

Oxygen fluorides are compounds of elements oxygen and fluorine with the general formula OnF2, where n = 1 to 6. Many different oxygen fluorides are known:
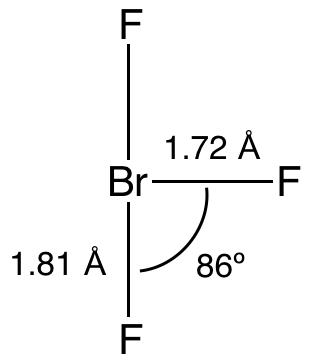
Bromine trifluoride is an interhalogen compound with the formula BrF3. At room temperature, it is a straw-coloured liquid with a pungent odor which decomposes violently on contact with water and organic compounds. It is a powerful fluorinating agent and an ionizing inorganic solvent. It is used to produce uranium hexafluoride (UF6) in the processing and reprocessing of nuclear fuel.

In the area of solid state chemistry. graphite intercalation compounds are materials prepared by intercalation of diverse guests into graphite. The materials have the formula (guest)Cn where n can range from 8 to 40's. The distance between the carbon layers increases significantly upon insertion of the guests. Common guests are reducing agents such as alkali metals. Strong oxidants, such as arsenic pentafluoride also intercalate into graphite. Intercalation involves electron transfer into or out of the host. The properties of these materials differ from those of the parent graphite.
Bromine compounds are compounds containing the element bromine (Br). These compounds usually form the -1, +1, +3 and +5 oxidation states. Bromine is intermediate in reactivity between chlorine and iodine, and is one of the most reactive elements. Bond energies to bromine tend to be lower than those to chlorine but higher than those to iodine, and bromine is a weaker oxidising agent than chlorine but a stronger one than iodine. This can be seen from the standard electrode potentials of the X2/X− couples (F, +2.866 V; Cl, +1.395 V; Br, +1.087 V; I, +0.615 V; At, approximately +0.3 V). Bromination often leads to higher oxidation states than iodination but lower or equal oxidation states to chlorination. Bromine tends to react with compounds including M–M, M–H, or M–C bonds to form M–Br bonds.
Organofluorine chemistry describes the chemistry of organofluorine compounds, organic compounds that contain a carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from oil and water repellents to pharmaceuticals, refrigerants, and reagents in catalysis. In addition to these applications, some organofluorine compounds are pollutants because of their contributions to ozone depletion, global warming, bioaccumulation, and toxicity. The area of organofluorine chemistry often requires special techniques associated with the handling of fluorinating agents.
Magnesium/Teflon/Viton (MTV) is a pyrolant. Teflon and Viton are trademarks of DuPont for polytetrafluoroethylene, (C2F4)n, and fluoroelastomer, (CH2CF2)n(CF(CF3)CF2)n.
A pyrolant is an energetic material that generates hot flames upon combustion. Pyrolants are metal-based pyrotechnic compositions containing virtually any oxidizer. The term was originally coined by Kuwahara in 1992, in a paper on magnesium/Teflon/Viton, to distinguish between compositions that serve as propellants and those yielding hot flames which are not necessarily suitable for propellant purposes.

Fluorine is a chemical element; it has symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. Fluorine is extremely reactive, as it reacts with all other elements except for the light inert gases.
Boron monofluoride or fluoroborylene is a chemical compound with the formula BF, one atom of boron and one of fluorine. It is an unstable gas, but it is a stable ligand on transition metals, in the same way as carbon monoxide. It is a subhalide, containing fewer than the normal number of fluorine atoms, compared with boron trifluoride. It can also be called a borylene, as it contains boron with two unshared electrons. BF is isoelectronic with carbon monoxide and dinitrogen; each molecule has 14 electrons.
A monofluoride is a chemical compound with one fluoride per formula unit. For a binary compound, this is the formula XF.

Fluorographene (or perfluorographane, graphene fluoride) is a fluorocarbon derivative of graphene. It is a two dimensional carbon sheet of sp3 hybridized carbons, with each carbon atom bound to one fluorine. The chemical formula is (CF)n. In comparison, Teflon (polytetrafluoroethylene), -(CF2)n-, consists of carbon "chains" with each carbon bound to two fluorines.
Fluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a higher order bond exist. Fluoride may act as a bridging ligand between two metals in some complex molecules. Molecules containing fluorine may also exhibit hydrogen bonding. Fluorine's chemistry includes inorganic compounds formed with hydrogen, metals, nonmetals, and even noble gases; as well as a diverse set of organic compounds. For many elements the highest known oxidation state can be achieved in a fluoride. For some elements this is achieved exclusively in a fluoride, for others exclusively in an oxide; and for still others the highest oxidation states of oxides and fluorides are always equal.

Fluorine azide or triazadienyl fluoride is a yellow green gas composed of nitrogen and fluorine with formula FN3. Its properties resemble those of ClN3, BrN3, and IN3. The bond between the fluorine atom and the nitrogen is very weak, leading to this substance being very unstable and prone to explosion. Calculations show the F–N–N angle to be around 102° with a straight line of 3 nitrogen atoms.











