Related Research Articles

Dynamite is an explosive made of nitroglycerin, sorbents, and stabilizers. It was invented by the Swedish chemist and engineer Alfred Nobel in Geesthacht, Northern Germany, and was patented in 1867. It rapidly gained wide-scale use as a more robust alternative to the traditional black powder explosives. It allows the use of nitroglycerine's favorable explosive properties while greatly reducing its risk of accidental detonation.

explosives description

Gunpowder, also commonly known as black powder to distinguish it from modern smokeless powder, is the earliest known chemical explosive. It consists of a mixture of sulfur, carbon and potassium nitrate (saltpeter). The sulfur and carbon act as fuels while the saltpeter is an oxidizer. Gunpowder has been widely used as a propellant in firearms, artillery, rocketry, and pyrotechnics, including use as a blasting agent for explosives in quarrying, mining, building pipelines and road building.

Nitrate is a polyatomic ion with the chemical formula NO−
3. Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives. Almost all inorganic nitrates are soluble in water. An example of an insoluble nitrate is bismuth oxynitrate.
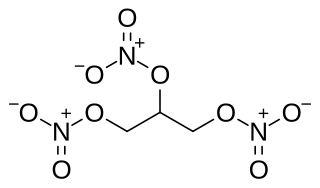
Nitroglycerin (NG), also known as trinitroglycerin (TNG), nitro, glyceryl trinitrate (GTN), or 1,2,3-trinitroxypropane, is a dense, colorless, oily, explosive liquid most commonly produced by nitrating glycerol with white fuming nitric acid under conditions appropriate to the formation of the nitric acid ester. Chemically, the substance is an organic nitrate compound rather than a nitro compound, but the traditional name is retained. Discovered in 1847 by Ascanio Sobrero, nitroglycerin has been used ever since as an active ingredient in the manufacture of explosives, namely dynamite, and as such it is employed in the construction, demolition, and mining industries. It is combined with nitrocellulose to form double-based smokeless powder, which has been used as a propellant in artillery and firearms since the 1880's.

Trinitrotoluene, more commonly known as TNT, more specifically 2,4,6-trinitrotoluene, and by its preferred IUPAC name 2-methyl-1,3,5-trinitrobenzene, is a chemical compound with the formula C6H2(NO2)3CH3. TNT is occasionally used as a reagent in chemical synthesis, but it is best known as an explosive material with convenient handling properties. The explosive yield of TNT is considered to be the standard comparative convention of bombs and asteroid impacts. In chemistry, TNT is used to generate charge transfer salts.

Potassium nitrate is a chemical compound with a sharp, salty, bitter taste and the chemical formula KNO
3. It is an ionic salt of potassium ions K+ and nitrate ions NO3−, and is therefore an alkali metal nitrate. It occurs in nature as a mineral, niter (or nitre in the UK). It is a source of nitrogen, and nitrogen was named after niter. Potassium nitrate is one of several nitrogen-containing compounds collectively referred to as saltpeter (or saltpetre in the UK).

Sodium nitrate is the chemical compound with the formula NaNO
3. This alkali metal nitrate salt is also known as Chile saltpeter to distinguish it from ordinary saltpeter, potassium nitrate. The mineral form is also known as nitratine, nitratite or soda niter.
Gelignite, also known as blasting gelatin or simply "jelly", is an explosive material consisting of collodion-cotton dissolved in either nitroglycerine or nitroglycol and mixed with wood pulp and saltpetre.
Nitromethane, sometimes shortened to simply "nitro", is an organic compound with the chemical formula CH
3NO
2. It is the simplest organic nitro compound. It is a polar liquid commonly used as a solvent in a variety of industrial applications such as in extractions, as a reaction medium, and as a cleaning solvent. As an intermediate in organic synthesis, it is used widely in the manufacture of pesticides, explosives, fibers, and coatings. Nitromethane is used as a fuel additive in various motorsports and hobbies, e.g. Top Fuel drag racing and miniature internal combustion engines in radio control, control line and free flight model aircraft.

Soda bread is a variety of quick bread traditionally made in a variety of cuisines in which sodium bicarbonate is used as a leavening agent instead of the traditional yeast. The ingredients of traditional soda bread are flour, baking soda, salt, and buttermilk. The buttermilk in the dough contains lactic acid, which reacts with the baking soda to form tiny bubbles of carbon dioxide. Other ingredients can be added, such as butter, egg, raisins, or nuts. An advantage of quick breads is their ability to be prepared quickly and reliably, without requiring the time-consuming skilled labor and temperature control needed for traditional yeast breads.

In organic chemistry, nitro compounds are organic compounds that contain one or more nitro functional groups. The nitro group is one of the most common explosophores used globally. The nitro group is also strongly electron-withdrawing. Because of this property, C−H bonds alpha (adjacent) to the nitro group can be acidic. For similar reasons, the presence of nitro groups in aromatic compounds retards electrophilic aromatic substitution but facilitates nucleophilic aromatic substitution. Nitro groups are rarely found in nature. They are almost invariably produced by nitration reactions starting with nitric acid.

Styphnic acid, or 2,4,6-trinitro-1,3-benzenediol, is a yellow astringent acid that forms hexagonal crystals. It is used in the manufacture of dyes, pigments, inks, medicines, and explosives such as lead styphnate. It is itself a low sensitivity explosive, similar to picric acid, but explodes upon rapid heating.
An Oxyliquit, also called liquid air explosive or liquid oxygen explosive, is an explosive material which is a mixture of liquid oxygen (LOX) with a suitable fuel, such as carbon, or an organic chemical, wood meal, or aluminium powder or sponge. It is a class of Sprengel explosives.
Pyrotol was an explosive available for a time after World War I. It was reprocessed from military surplus, with a typical composition of 60% smokeless powder, 34% sodium nitrate, and 6% of 40% nitroglycerin dynamite. Usually used in combination with dynamite, it created an incendiary blast. Since it was very inexpensive, it was often used by farmers to remove tree stumps and clear ditches. The substance was known for being used to commit the Bath School bombing in 1927. Distribution of pyrotol for farm use was discontinued in 1928, due to exhaustion of the supply of surplus explosives.

Silver azide is the chemical compound with the formula AgN3. It is a silver(I) salt of hydrazoic acid. It forms a colorless crystals. Like most azides, it is a primary explosive.

Tovex is a water-gel explosive composed of ammonium nitrate and methylammonium nitrate that has several advantages over traditional dynamite, including lower toxicity and safer manufacture, transport, and storage. It has thus almost entirely replaced dynamite. There are numerous versions ranging from shearing charges to aluminized common blasting agents. Tovex is used by 80% of international oil companies for seismic exploration.
Explosive materials are produced in numerous physical forms for their use in mining, engineering, or military applications. The different physical forms and fabrication methods are grouped together in several use forms of explosives.

A water-gel explosive is a fuel sensitized explosive mixture consisting of an aqueous ammonium nitrate solution that acts as the oxidizer. Water gels that are cap-insensitive are referred to under United States safety regulations as blasting agents. Water gel explosives have a jelly-like consistency and come in sausage-like packing stapled shut on both sides.
Nitroxylic acid or hydronitrous acid is an unstable reduced oxonitrogen acid. It has formula H4N2O4 containing nitrogen in the +2 oxidation state. The corresponding anion called nitroxylate is N
2O4−
4 or NO2−
2.