Related Research Articles

Isotope analysis is the identification of isotopic signature, abundance of certain stable isotopes of chemical elements within organic and inorganic compounds. Isotopic analysis can be used to understand the flow of energy through a food web, to reconstruct past environmental and climatic conditions, to investigate human and animal diets, for food authentification, and a variety of other physical, geological, palaeontological and chemical processes. Stable isotope ratios are measured using mass spectrometry, which separates the different isotopes of an element on the basis of their mass-to-charge ratio.
Isotope geochemistry is an aspect of geology based upon the study of natural variations in the relative abundances of isotopes of various elements. Variations in isotopic abundance are measured by isotope ratio mass spectrometry, and can reveal information about the ages and origins of rock, air or water bodies, or processes of mixing between them.
Isotopic labeling is a technique used to track the passage of an isotope through a reaction, metabolic pathway, or cell. The reactant is 'labeled' by replacing specific atoms by their isotope. The reactant is then allowed to undergo the reaction. The position of the isotopes in the products is measured to determine the sequence the isotopic atom followed in the reaction or the cell's metabolic pathway. The nuclides used in isotopic labeling may be stable nuclides or radionuclides. In the latter case, the labeling is called radiolabeling.

The term freshet is most commonly used to describe a snowmelt, an annual high water event on rivers resulting from snow and river ice melting. A spring freshet can sometimes last several weeks on large river systems, resulting in significant inundation of flood plains as the snowpack melts in the river's watershed. Freshets can occur with differing strength and duration depending upon the depth of the snowpack and the local average rates of warming temperatures. Deeper snowpacks which melt quickly can result in more severe flooding. Late spring melts allow for faster flooding; this is because the relatively longer days and higher solar angle allow for average melting temperatures to be reached quickly, causing snow to melt rapidly. Snowpacks at higher altitudes and in mountainous areas remain cold and tend to melt over a longer period of time and thus do not contribute to major flooding. Serious flooding from southern freshets are more often related to rain storms of large tropical weather systems rolling in from the South Atlantic or Gulf of Mexico, to add their powerful heating capacity to lesser snow packs. Tropically induced rainfall influenced quick melts can also affect snow cover to latitudes as far north as southern Canada, so long as the generally colder air mass is not blocking northward movement of low pressure systems.
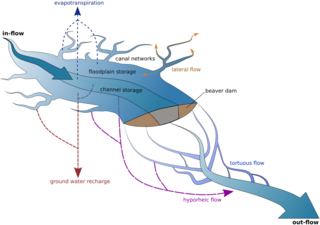
Ecohydrology is an interdisciplinary scientific field studying the interactions between water and ecological systems. It is considered a sub discipline of hydrology, with an ecological focus. These interactions may take place within water bodies, such as rivers and lakes, or on land, in forests, deserts, and other terrestrial ecosystems. Areas of research in ecohydrology include transpiration and plant water use, adaption of organisms to their water environment, influence of vegetation and benthic plants on stream flow and function, and feedbacks between ecological processes, the soil carbon sponge and the hydrological cycle.
Isotope hydrology is a field of geochemistry and hydrology that uses naturally occurring stable and radioactive isotopic techniques to evaluate the age and origins of surface and groundwater and the processes within the atmospheric hydrologic cycle. Isotope hydrology applications are highly diverse, and used for informing water-use policy, mapping aquifers, conserving water supplies, assessing sources of water pollution, and increasingly are used in eco-hydrology to study human impacts on all dimensions of the hydrological cycle and ecosystem services.
An isotopic signature is a ratio of non-radiogenic 'stable isotopes', stable radiogenic isotopes, or unstable radioactive isotopes of particular elements in an investigated material. The ratios of isotopes in a sample material are measured by isotope-ratio mass spectrometry against an isotopic reference material. This process is called isotope analysis.
The hyporheic zone is the region of sediment and porous space beneath and alongside a stream bed, where there is mixing of shallow groundwater and surface water. The flow dynamics and behavior in this zone is recognized to be important for surface water/groundwater interactions, as well as fish spawning, among other processes. As an innovative urban water management practice, the hyporheic zone can be designed by engineers and actively managed for improvements in both water quality and riparian habitat.
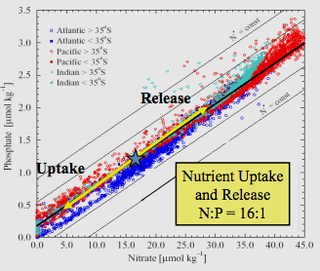
The Redfield ratio or Redfield stoichiometry is the consistent atomic ratio of carbon, nitrogen and phosphorus found in marine phytoplankton and throughout the deep oceans.
An isoscape is a geologic map of isotope distribution. It is a spatially explicit prediction of elemental isotope ratios (δ) that is produced by executing process-level models of elemental isotope fractionation or distribution in a geographic information system (GIS).

In hydrology, stemflow is the flow of intercepted water down the trunk or stem of a plant. Stemflow, along with throughfall, is responsible for the transferral of precipitation and nutrients from the canopy to the soil. In tropical rainforests, where this kind of flow can be substantial, erosion gullies can form at the base of the trunk. However, in more temperate climates stemflow levels are low and have little erosional power.
Baseflow is the portion of the streamflow that is sustained between precipitation events, fed to streams by delayed pathways. It should not be confused with groundwater flow. Fair weather flow is also called base flow.
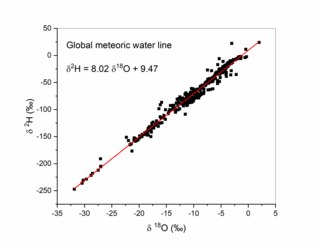
The Global Meteoric Water Line (GMWL) describes the global annual average relationship between hydrogen and oxygen isotope (Oxygen-18 and Deuterium) ratios in natural meteoric waters. The GMWL was first developed in 1961 by Harmon Craig, and has subsequently been widely used to track water masses in environmental geochemistry and hydrogeology.

A hydrologic model is a simplification of a real-world system that aids in understanding, predicting, and managing water resources. Both the flow and quality of water are commonly studied using hydrologic models.
Thure E. Cerling is a Distinguished Professor of Geology and Geophysics and a Distinguished Professor of Biology at the University of Utah. Cerling is a leading expert in the evolution of modern landscapes including modern mammals and their associated grassland ecologies and stable isotope analyses of the atmosphere. Cerling lives in Salt Lake City, Utah.

The term stable isotope has a meaning similar to stable nuclide, but is preferably used when speaking of nuclides of a specific element. Hence, the plural form stable isotopes usually refers to isotopes of the same element. The relative abundance of such stable isotopes can be measured experimentally, yielding an isotope ratio that can be used as a research tool. Theoretically, such stable isotopes could include the radiogenic daughter products of radioactive decay, used in radiometric dating. However, the expression stable-isotope ratio is preferably used to refer to isotopes whose relative abundances are affected by isotope fractionation in nature. This field is termed stable isotope geochemistry.
Isotopic reference materials are compounds with well-defined isotopic compositions and are the ultimate sources of accuracy in mass spectrometric measurements of isotope ratios. Isotopic references are used because mass spectrometers are highly fractionating. As a result, the isotopic ratio that the instrument measures can be very different from that in the sample's measurement. Moreover, the degree of instrument fractionation changes during measurement, often on a timescale shorter than the measurement's duration, and can depend on the characteristics of the sample itself. By measuring a material of known isotopic composition, fractionation within the mass spectrometer can be removed during post-measurement data processing. Without isotope references, measurements by mass spectrometry would be much less accurate and could not be used in comparisons across different analytical facilities. Due to their critical role in measuring isotope ratios, and in part, due to historical legacy, isotopic reference materials define the scales on which isotope ratios are reported in the peer-reviewed scientific literature.

Amy Townsend-Small is the director of the Environmental Studies Program as well as an associate professor in the Department of Geology and Geography at the University of Cincinnati.

Hendratta Ali is a geoscientist who does work in hydrology, aqueous geochemistry, exploration geology and equity geoscience. Her home institution is the Department of Geosciences at Fort Hays State University. She was awarded the 2021 Geological Society of America Randolph Bromery award and Fort Hays State University President’s Distinguished Scholar Award. Ali is a native of Cameroon.
Elizabeth A. Canuel is a chemical oceanographer known for her work on organic carbon cycling in aquatic environments. She is the Chancellor Professor of Marine Science at the College of William & Mary and is an elected fellow of the Geochemical Society and the European Association of Geochemistry.
References
- 1 2 3 "Carol Kendall". www.usgs.gov. Retrieved 2021-08-23.
- ↑ Kendall, Carol (1976). Petrology and stable isotope geochemistry of three wells in the Buttes Area of the Salton Sea geothermal field, Imperial Valley, California, U.S.A. (Thesis). Riverside, Calif. OCLC 4428861.
- 1 2 "Distinguished Alumni | Department of Geology | University of Maryland". www.geol.umd.edu. Retrieved 2021-08-23.
- ↑ Kendall, Carol (1993). Impact of isotopic heterogeneity in shallow systems on modeling of stormflow generation (Thesis). OCLC 30104048.
- ↑ Kendall, Carol.; Coplen, Tyler B. (1985-06-01). "Multi-sample conversion of water to hydrogen by zinc for stable isotope determination". Analytical Chemistry. 57 (7): 1437–1440. doi:10.1021/ac00284a058. ISSN 0003-2700.
- ↑ Pellerin, Brian A.; Downing, Bryan D.; Kendall, Carol; Dahlgren, Randy A.; Kraus, Tamara E. C.; Saraceno, Johnfranco; Spencer, Robert G. M.; Bergamaschi, Brian A. (2009). "Assessing the sources and magnitude of diurnal nitrate variability in the San Joaquin River (California) with an in situ optical nitrate sensor and dual nitrate isotopes". Freshwater Biology. 54 (2): 376–387. doi:10.1111/j.1365-2427.2008.02111.x.
- ↑ Carol Kendall, Megan B. Young (2015), "Isotope data, chemical data, isotope techniques, nitrate, ammonium, Sacramento river, miner slough, steamboat slough, organic matter, yolo slough, cache slough, phytoplankton, San Francisco Estuary, isotopic composition", Tracing nutrient and organic matter sources and biogeochemical processes in the Sacramento River and Northern Delta: proof of concept using stable isotope data, U.S. Geological Survey, doi:10.5066/f7qj7fcm , retrieved 2021-08-24
- ↑ Wankel, Scott D.; Kendall, Carol; Francis, Chris A.; Paytan, Adina (2006). "Nitrogen sources and cycling in the San Francisco Bay Estuary: A nitrate dual isotopic composition approach". Limnology and Oceanography. 51 (4): 1654–1664. Bibcode:2006LimOc..51.1654W. doi:10.4319/lo.2006.51.4.1654. ISSN 1939-5590. S2CID 2670883.
- ↑ Kendall, Carol; Coplen, Tyler B. (2001). "Distribution of oxygen-18 and deuterium in river waters across the United States". Hydrological Processes. 15 (7): 1363–1393. Bibcode:2001HyPr...15.1363K. doi:10.1002/hyp.217. ISSN 0885-6087. S2CID 27744095.
- ↑ Kendall, Carol; Silva, Steven R.; Kelly, Valerie J. (2001). "Carbon and nitrogen isotopic compositions of particulate organic matter in four large river systems across the United States". Hydrological Processes. 15 (7): 1301–1346. Bibcode:2001HyPr...15.1301K. doi:10.1002/hyp.216. ISSN 0885-6087. S2CID 129144544.
- ↑ Amundson, Ronald; Austin, A. T.; Schuur, E. A. G.; Yoo, K.; Matzek, V.; Kendall, C.; Uebersax, A.; Brenner, D.; Baisden, W. T. (2003). "Global patterns of the isotopic composition of soil and plant nitrogen: GLOBAL SOIL AND PLANT N ISOTOPES". Global Biogeochemical Cycles. 17 (1). doi:10.1029/2002GB001903. S2CID 129960646.
- ↑ Doctor, Daniel H.; Kendall, Carol; Sebestyen, Stephen D.; Shanley, James B.; Ohte, Nobuhito; Boyer, Elizabeth W. (2008-07-01). "Carbon isotope fractionation of dissolved inorganic carbon (DIC) due to outgassing of carbon dioxide from a headwater stream". Hydrological Processes. 22 (14): 2410–2423. Bibcode:2008HyPr...22.2410D. doi:10.1002/hyp.6833. S2CID 27087096.
- ↑ Burns, Douglas A.; Kendall, Carol (2002). "Analysis of δ 15 N and δ 18 O to differentiate NO 3 − sources in runoff at two watersheds in the Catskill Mountains of New York: DUAL-ISOTOPE ANALYSIS". Water Resources Research. 38 (5): 9–1–9–11. doi:10.1029/2001WR000292. S2CID 128953208.
- ↑ Isotope Tracers in Catchment Hydrology. Elsevier. 1998. p. 816. ISBN 9780080929156.
- ↑ "Kendall". Honors Program. Retrieved 21 August 2021.
- ↑ "Walter Langbein Lecture". www.agu.org. Retrieved 2021-08-23.