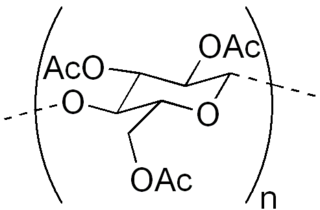A carboxylic acid is an organic acid that contains a carboxyl group (C(=O)OH) attached to an R-group. The general formula of a carboxylic acid is R−COOH or R−CO2H, with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion.

An ester is a chemical compound derived from an oxoacid in which at least one –OH hydroxyl group is replaced by an –O– alkyl (alkoxy) group, as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils.

A tablet is a pharmaceutical oral dosage form or solid unit dosage form. Tablets may be defined as the solid unit dosage form of medicament or medicaments with suitable excipients. It comprises a mixture of active substances and excipients, usually in powder form, pressed or compacted from a powder into a solid dose.
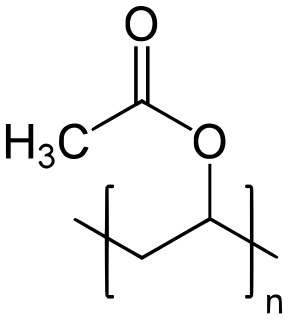
Polyvinyl acetate (PVA, PVAc, poly(ethenyl ethanoate)), commonly known as wood glue, PVA glue, white glue, carpenter's glue, school glue, or Elmer's glue in the US, is a widely available adhesive used for porous materials like wood, paper, and cloth. An aliphatic rubbery synthetic polymer with the formula (C4H6O2)n, it belongs to the polyvinyl ester family, with the general formula −[RCOOCHCH2]−. It is a type of thermoplastic.

Phthalic anhydride is the organic compound with the formula C6H4(CO)2O. It is the anhydride of phthalic acid. Phthalic anhydride is a principal commercial form of phthalic acid. It was the first anhydride of a dicarboxylic acid to be used commercially. This white solid is an important industrial chemical, especially for the large-scale production of plasticizers for plastics. In 2000, the worldwide production volume was estimated to be about 3 million tonnes per year.

Cellulose acetate refers to any acetate ester of cellulose, usually cellulose diacetate. It was first prepared in 1865. A bioplastic, cellulose acetate is used as a film base in photography, as a component in some coatings, and as a frame material for eyeglasses; it is also used as a synthetic fiber in the manufacture of cigarette filters and playing cards. In photographic film, cellulose acetate film replaced nitrate film in the 1950s, being far less flammable and cheaper to produce.

Malonic acid (IUPAC systematic name: propanedioic acid) is a dicarboxylic acid with structure CH2(COOH)2. The ionized form of malonic acid, as well as its esters and salts, are known as malonates. For example, diethyl malonate is malonic acid's diethyl ester. The name originates from the Greek word μᾶλον (malon) meaning 'apple'.
An excipient is a substance formulated alongside the active ingredient of a medication, included for the purpose of long-term stabilization, bulking up solid formulations that contain potent active ingredients in small amounts, or to confer a therapeutic enhancement on the active ingredient in the final dosage form, such as facilitating drug absorption, reducing viscosity, or enhancing solubility. Excipients can also be useful in the manufacturing process, to aid in the handling of the active substance concerns such as by facilitating powder flowability or non-stick properties, in addition to aiding in vitro stability such as prevention of denaturation or aggregation over the expected shelf life. The selection of appropriate excipients also depends upon the route of administration and the dosage form, as well as the active ingredient and other factors. A comprehensive classification system based on structure-property-application relationships has been proposed for excipients used in parenteral medications.

Methyl acetate, also known as MeOAc, acetic acid methyl ester or methyl ethanoate, is a carboxylate ester with the formula CH3COOCH3. It is a flammable liquid with a characteristically pleasant smell reminiscent of some glues and nail polish removers. Methyl acetate is occasionally used as a solvent, being weakly polar and lipophilic, but its close relative ethyl acetate is a more common solvent being less toxic and less soluble in water. Methyl acetate has a solubility of 25% in water at room temperature. At elevated temperature its solubility in water is much higher. Methyl acetate is not stable in the presence of strong aqueous bases or aqueous acids. Methyl acetate is not considered a VOC in the USA.

Terephthalic acid is an organic compound with formula C6H4(CO2H)2. This white solid is a commodity chemical, used principally as a precursor to the polyester PET, used to make clothing and plastic bottles. Several million tonnes are produced annually. The common name is derived from the turpentine-producing tree Pistacia terebinthus and phthalic acid.
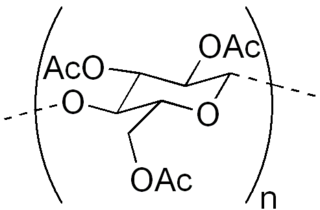
Cellulose triacetate, is a chemical compound produced from cellulose and a source of acetate esters, typically acetic anhydride. Triacetate is commonly used for the creation of fibres and film base. It is chemically similar to cellulose acetate. Its distinguishing characteristic is that in triacetate, at least "92 percent of the hydroxyl groups are acetylated." During the manufacture of triacetate, the cellulose is completely acetylated; whereas in normal cellulose acetate or cellulose diacetate, it is only partially acetylated. Triacetate is significantly more heat resistant than cellulose acetate.

Acetone, propanone or dimethyl ketone, is an organic compound with the formula (CH3)2CO. It is the simplest and smallest ketone. It is a colourless, highly volatile and flammable liquid with a characteristic pungent odour.
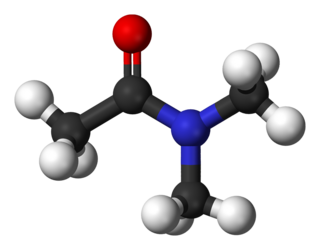
Dimethylacetamide (DMAc or DMA) is the organic compound with the formula CH3C(O)N(CH3)2. This colorless, water-miscible, high-boiling liquid is commonly used as a polar solvent in organic synthesis. DMA is miscible with most other solvents, although it is poorly soluble in aliphatic hydrocarbons.
An enteric coating is a polymer barrier applied to oral medication that prevents its dissolution or disintegration in the gastric environment. This helps by either protecting drugs from the acidity of the stomach, the stomach from the detrimental effects of the drug, or to release the drug after the stomach. Some drugs are unstable at the pH of gastric acid and need to be protected from degradation. Enteric coating is also an effective method to obtain drug targeting. Other drugs such as some anthelmintics may need to reach a high concentration in a specific part of the intestine. Enteric coating may also be used during studies as a research tool to determine drug absorption. Enteric-coated medications pertain to the "delayed action" dosage form category. Tablets, mini-tablets, pellets and granules are the most common enteric-coated dosage forms.
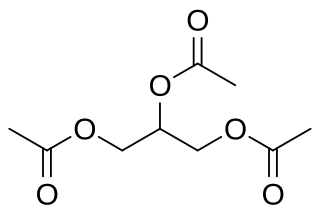
Triacetin, is the organic compound with the formula C3H5(OCOCH3)3. It is classified as a triglyceride, i.e., the triester of glycerol. It is a colorless, viscous, and odorless liquid with a high boiling point and a low melting point. It has a mild, sweet taste in concentrations lower than 500 ppm, but may appear bitter at higher concentrations. It is one of the glycerine acetate compounds.
Solution polymerization is a method of industrial polymerization. In this procedure, a monomer is dissolved in a non-reactive solvent that contains a catalyst or initiator.

Thin-film drug delivery uses a dissolving film or oral drug strip to administer drugs via absorption in the mouth and/or via the small intestines (enterically). A film is prepared using hydrophilic polymers that rapidly dissolves on the tongue or buccal cavity, delivering the drug to the systemic circulation via dissolution when contact with liquid is made.

Acetic acid, systematically named ethanoic acid, is an acidic, colourless liquid and organic compound with the chemical formula CH3COOH. Vinegar is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water and other trace elements.
Polyvinyl acetate phthalate (PVAP) is a commonly used polymer phthalate in the formulation of pharmaceuticals, such as the enteric coating of tablets or capsules. It is a vinyl acetate polymer that is partially hydrolyzed and then esterified with phthalic acid. Its main use in pharmaceutics is with enteric formulations and controlled release formulations.
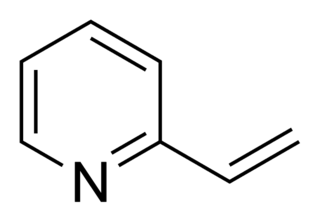
2-Vinylpyridine is an organic compound with the formula CH2CHC5H4N. It is a derivative of pyridine with a vinyl group in the 2-position, next to the nitrogen. It is a colorless liquid, although samples are often brown. It is used industrially as a precursor to specialty polymers and as an intermediate in the chemical, pharmaceutical, dye, and photo industries. Vinylpyridine is sensitive to polymerization. It may be stabilized with a free radical inhibitor such as tert-butylcatechol. Owing to its tendency to polymerize, samples are typically refrigerated.