In a chemical reaction, chemical equilibrium is the state in which both the reactants and products are present in concentrations which have no further tendency to change with time, so that there is no observable change in the properties of the system. This state results when the forward reaction proceeds at the same rate as the reverse reaction. The reaction rates of the forward and backward reactions are generally not zero, but they are equal. Thus, there are no net changes in the concentrations of the reactants and products. Such a state is known as dynamic equilibrium.
In chemistry, biochemistry, and pharmacology, a dissociation constant is a specific type of equilibrium constant that measures the propensity of a larger object to separate (dissociate) reversibly into smaller components, as when a complex falls apart into its component molecules, or when a salt splits up into its component ions. The dissociation constant is the inverse of the association constant. In the special case of salts, the dissociation constant can also be called an ionization constant. For a general reaction:

In chemistry, pH (, denoting "potential of hydrogen" or "power of hydrogen") is a scale used to specify the acidity or basicity of an aqueous solution. Acidic solutions (solutions with higher concentrations of H+ ions) are measured to have lower pH values than basic or alkaline solutions.
A buffer solution is an aqueous solution consisting of a mixture of a weak acid and its conjugate base, or vice versa. Its pH changes very little when a small amount of strong acid or base is added to it. Buffer solutions are used as a means of keeping pH at a nearly constant value in a wide variety of chemical applications. In nature, there are many systems that use buffering for pH regulation. For example, the bicarbonate buffering system is used to regulate the pH of blood, and bicarbonate also acts as a buffer in the ocean.
An acid dissociation constant, Ka, is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction
Solubility equilibrium is a type of dynamic equilibrium that exists when a chemical compound in the solid state is in chemical equilibrium with a solution of that compound. The solid may dissolve unchanged, with dissociation or with chemical reaction with another constituent of the solution, such as acid or alkali. Each solubility equilibrium is characterized by a temperature-dependent solubility product which functions like an equilibrium constant. Solubility equilibria are important in pharmaceutical, environmental and many other scenarios.
In electrochemistry, the Nernst equation is an equation that relates the reduction potential of a reaction to the standard electrode potential, temperature, and activities of the chemical species undergoing reduction and oxidation. It was named after Walther Nernst, a German physical chemist who formulated the equation.
A pH indicator is a halochromic chemical compound added in small amounts to a solution so the pH (acidity or basicity) of the solution can be determined visually. Hence, a pH indicator is a chemical detector for hydronium ions (H3O+) or hydrogen ions (H+) in the Arrhenius model. Normally, the indicator causes the color of the solution to change depending on the pH. Indicators can also show change in other physical properties; for example, olfactory indicators show change in their odor. The pH value of a neutral solution is 7.0 at 25°C (standard laboratory conditions). Solutions with a pH value below 7.0 are considered acidic and solutions with pH value above 7.0 are basic (alkaline). As most naturally occurring organic compounds are weak protolytes, carboxylic acids and amines, pH indicators find many applications in biology and analytical chemistry. Moreover, pH indicators form one of the three main types of indicator compounds used in chemical analysis. For the quantitative analysis of metal cations, the use of complexometric indicators is preferred, whereas the third compound class, the redox indicators, are used in titrations involving a redox reaction as the basis of the analysis.
The self-ionization of water (also autoionization of water, and autodissociation of water) is an ionization reaction in pure water or in an aqueous solution, in which a water molecule, H2O, deprotonates (loses the nucleus of one of its hydrogen atoms) to become a hydroxide ion, OH−. The hydrogen nucleus, H+, immediately protonates another water molecule to form hydronium, H3O+. It is an example of autoprotolysis, and exemplifies the amphoteric nature of water.
In chemistry and biochemistry, the Henderson–Hasselbalch equation

Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent variable and the pH of the solution as the dependent variable.

An acid–base titration is a method of quantitative analysis for determining the concentration of an acid or base by exactly neutralizing it with a standard solution of base or acid having known concentration. A pH indicator is used to monitor the progress of the acid–base reaction. If the acid dissociation constant (pKa) of the acid or base dissociation constant (pKb) of base in the analyte solution is known, its solution concentration (molarity) can be determined. Alternately, the pKa can be determined if the analyte solution has a known solution concentration by constructing a titration curve.
The equilibrium constant of a chemical reaction is the value of its reaction quotient at chemical equilibrium, a state approached by a dynamic chemical system after sufficient time has elapsed at which its composition has no measurable tendency towards further change. For a given set of reaction conditions, the equilibrium constant is independent of the initial analytical concentrations of the reactant and product species in the mixture. Thus, given the initial composition of a system, known equilibrium constant values can be used to determine the composition of the system at equilibrium. However, reaction parameters like temperature, solvent, and ionic strength may all influence the value of the equilibrium constant.
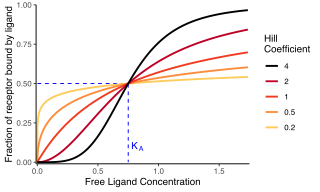
In biochemistry and pharmacology, the Hill equation refers to two closely related equations that reflect the binding of ligands to macromolecules, as a function of the ligand concentration. A ligand is "a substance that forms a complex with a biomolecule to serve a biological purpose", and a macromolecule is a very large molecule, such as a protein, with a complex structure of components. Protein-ligand binding typically changes the structure of the target protein, thereby changing its function in a cell.
An ICE table or RICE box or RICE chart is a tabular system of keeping track of changing concentrations in an equilibrium reaction. ICE stands for initial, change, equilibrium. It is used in chemistry to keep track of the changes in amount of substance of the reactants and also organize a set of conditions that one wants to solve with. Some sources refer to a RICE table where the added R stands for the reaction to which the table refers.
Equilibrium constants are determined in order to quantify chemical equilibria. When an equilibrium constant K is expressed as a concentration quotient,
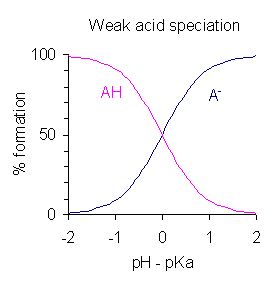
Speciation of ions refers to the changing concentration of varying forms of an ion as the pH of the solution changes.
A stability constant is an equilibrium constant for the formation of a complex in solution. It is a measure of the strength of the interaction between the reagents that come together to form the complex. There are two main kinds of complex: compounds formed by the interaction of a metal ion with a ligand and supramolecular complexes, such as host–guest complexes and complexes of anions. The stability constant(s) provide(s) the information required to calculate the concentration(s) of the complex(es) in solution. There are many areas of application in chemistry, biology and medicine.
Equilibrium chemistry is concerned with systems in chemical equilibrium. The unifying principle is that the free energy of a system at equilibrium is the minimum possible, so that the slope of the free energy with respect to the reaction coordinate is zero. This principle, applied to mixtures at equilibrium provides a definition of an equilibrium constant. Applications include acid–base, host–guest, metal–complex, solubility, partition, chromatography and redox equilibria.
Acid strength is the tendency of an acid, symbolised by the chemical formula , to dissociate into a proton, , and an anion, . The dissociation of a strong acid in solution is effectively complete, except in its most concentrated solutions.












