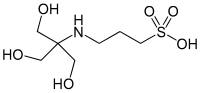
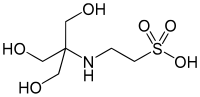
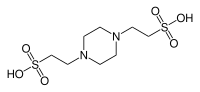
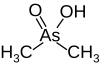
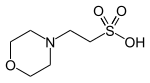
An acid is a molecule or ion capable of either donating a proton (i.e. hydrogen ion, H+), known as a Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis acid.

In chemistry, an acid–base reaction is a chemical reaction that occurs between an acid and a base. It can be used to determine pH via titration. Several theoretical frameworks provide alternative conceptions of the reaction mechanisms and their application in solving related problems; these are called the acid–base theories, for example, Brønsted–Lowry acid–base theory.
In a chemical reaction, chemical equilibrium is the state in which both the reactants and products are present in concentrations which have no further tendency to change with time, so that there is no observable change in the properties of the system. This state results when the forward reaction proceeds at the same rate as the reverse reaction. The reaction rates of the forward and backward reactions are generally not zero, but they are equal. Thus, there are no net changes in the concentrations of the reactants and products. Such a state is known as dynamic equilibrium.

In chemistry, pH, also referred to as acidity or basicity, historically denotes "potential of hydrogen". It is a logarithmic scale used to specify the acidity or basicity of aqueous solutions. Acidic solutions are measured to have lower pH values than basic or alkaline solutions.

Carbonic acid is a chemical compound with the chemical formula H2CO3. The molecule rapidly converts to water and carbon dioxide in the presence of water. However, in the absence of water, it is quite stable at room temperature. The interconversion of carbon dioxide and carbonic acid is related to the breathing cycle of animals and the acidification of natural waters.
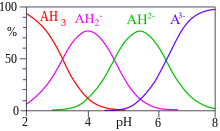
In chemistry, an acid dissociation constant is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction
Solubility equilibrium is a type of dynamic equilibrium that exists when a chemical compound in the solid state is in chemical equilibrium with a solution of that compound. The solid may dissolve unchanged, with dissociation, or with chemical reaction with another constituent of the solution, such as acid or alkali. Each solubility equilibrium is characterized by a temperature-dependent solubility product which functions like an equilibrium constant. Solubility equilibria are important in pharmaceutical, environmental and many other scenarios.
The self-ionization of water (also autoionization of water, and autodissociation of water, or simply dissociation of water) is an ionization reaction in pure water or in an aqueous solution, in which a water molecule, H2O, deprotonates (loses the nucleus of one of its hydrogen atoms) to become a hydroxide ion, OH−. The hydrogen nucleus, H+, immediately protonates another water molecule to form a hydronium cation, H3O+. It is an example of autoprotolysis, and exemplifies the amphoteric nature of water.
In chemistry, the common-ion effect refers to the decrease in solubility of an ionic precipitate by the addition to the solution of a soluble compound with an ion in common with the precipitate. This behaviour is a consequence of Le Chatelier's principle for the equilibrium reaction of the ionic association/dissociation. The effect is commonly seen as an effect on the solubility of salts and other weak electrolytes. Adding an additional amount of one of the ions of the salt generally leads to increased precipitation of the salt, which reduces the concentration of both ions of the salt until the solubility equilibrium is reached. The effect is based on the fact that both the original salt and the other added chemical have one ion in common with each other.
In chemistry and biochemistry, the Henderson–Hasselbalch equation

In chemistry, neutralization or neutralisation is a chemical reaction in which acid and a base react with an equivalent quantity of each other. In a reaction in water, neutralization results in there being no excess of hydrogen or hydroxide ions present in the solution. The pH of the neutralized solution depends on the acid strength of the reactants.
The equilibrium constant of a chemical reaction is the value of its reaction quotient at chemical equilibrium, a state approached by a dynamic chemical system after sufficient time has elapsed at which its composition has no measurable tendency towards further change. For a given set of reaction conditions, the equilibrium constant is independent of the initial analytical concentrations of the reactant and product species in the mixture. Thus, given the initial composition of a system, known equilibrium constant values can be used to determine the composition of the system at equilibrium. However, reaction parameters like temperature, solvent, and ionic strength may all influence the value of the equilibrium constant.
In thermodynamics, an activity coefficient is a factor used to account for deviation of a mixture of chemical substances from ideal behaviour. In an ideal mixture, the microscopic interactions between each pair of chemical species are the same and, as a result, properties of the mixtures can be expressed directly in terms of simple concentrations or partial pressures of the substances present e.g. Raoult's law. Deviations from ideality are accommodated by modifying the concentration by an activity coefficient. Analogously, expressions involving gases can be adjusted for non-ideality by scaling partial pressures by a fugacity coefficient.

Dissociation in chemistry is a general process in which molecules (or ionic compounds such as salts, or complexes) separate or split into other things such as atoms, ions, or radicals, usually in a reversible manner. For instance, when an acid dissolves in water, a covalent bond between an electronegative atom and a hydrogen atom is broken by heterolytic fission, which gives a proton (H+) and a negative ion. Dissociation is the opposite of association or recombination.
An ICE table or RICE box or RICE chart is a tabular system of keeping track of changing concentrations in an equilibrium reaction. ICE stands for initial, change, equilibrium. It is used in chemistry to keep track of the changes in amount of substance of the reactants and also organize a set of conditions that one wants to solve with. Some sources refer to a RICE table where the added R stands for the reaction to which the table refers. Others simply call it a concentration table.
Equilibrium constants are determined in order to quantify chemical equilibria. When an equilibrium constant K is expressed as a concentration quotient,
The Charlot equation, named after Gaston Charlot, is used in analytical chemistry to relate the hydrogen ion concentration, and therefore the pH, with the formal analytical concentration of an acid and its conjugate base. It can be used for computing the pH of buffer solutions when the approximations of the Henderson–Hasselbalch equation break down. The Henderson–Hasselbalch equation assumes that the autoionization of water is negligible and that the dissociation or hydrolysis of the acid and the base in solution are negligible.
In coordination chemistry, a stability constant is an equilibrium constant for the formation of a complex in solution. It is a measure of the strength of the interaction between the reagents that come together to form the complex. There are two main kinds of complex: compounds formed by the interaction of a metal ion with a ligand and supramolecular complexes, such as host–guest complexes and complexes of anions. The stability constant(s) provide(s) the information required to calculate the concentration(s) of the complex(es) in solution. There are many areas of application in chemistry, biology and medicine.
Equilibrium chemistry is concerned with systems in chemical equilibrium. The unifying principle is that the free energy of a system at equilibrium is the minimum possible, so that the slope of the free energy with respect to the reaction coordinate is zero. This principle, applied to mixtures at equilibrium provides a definition of an equilibrium constant. Applications include acid–base, host–guest, metal–complex, solubility, partition, chromatography and redox equilibria.
Acid strength is the tendency of an acid, symbolised by the chemical formula , to dissociate into a proton, , and an anion, . The dissociation of a strong acid in solution is effectively complete, except in its most concentrated solutions.