
Agarose gel electrophoresis is a method of gel electrophoresis used in biochemistry, molecular biology, genetics, and clinical chemistry to separate a mixed population of macromolecules such as DNA or proteins in a matrix of agarose, one of the two main components of agar. The proteins may be separated by charge and/or size, and the DNA and RNA fragments by length. Biomolecules are separated by applying an electric field to move the charged molecules through an agarose matrix, and the biomolecules are separated by size in the agarose gel matrix.

Agarose is a heteropolysaccharide, generally extracted from certain red seaweed. It is a linear polymer made up of the repeating unit of agarobiose, which is a disaccharide made up of D-galactose and 3,6-anhydro-L-galactopyranose. Agarose is one of the two principal components of agar, and is purified from agar by removing agar's other component, agaropectin.

Gel electrophoresis is a method for separation and analysis of biomacromolecules and their fragments, based on their size and charge. It is used in clinical chemistry to separate proteins by charge or size and in biochemistry and molecular biology to separate a mixed population of DNA and RNA fragments by length, to estimate the size of DNA and RNA fragments or to separate proteins by charge.
The isoelectric point (pI, pH(I), IEP), is the pH at which a molecule carries no net electrical charge or is electrically neutral in the statistical mean. The standard nomenclature to represent the isoelectric point is pH(I). However, pI is also used. For brevity, this article uses pI. The net charge on the molecule is affected by pH of its surrounding environment and can become more positively or negatively charged due to the gain or loss, respectively, of protons (H+).
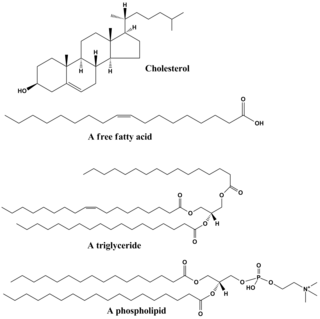
Lipids are a broad group of organic compounds which include fats, waxes, sterols, fat-soluble vitamins, monoglycerides, diglycerides, phospholipids, and others. The functions of lipids include storing energy, signaling, and acting as structural components of cell membranes. Lipids have applications in the cosmetic and food industries, and in nanotechnology.

Polyacrylamide gel electrophoresis (PAGE) is a technique widely used in biochemistry, forensic chemistry, genetics, molecular biology and biotechnology to separate biological macromolecules, usually proteins or nucleic acids, according to their electrophoretic mobility. Electrophoretic mobility is a function of the length, conformation, and charge of the molecule. Polyacrylamide gel electrophoresis is a powerful tool used to analyze RNA samples. When polyacrylamide gel is denatured after electrophoresis, it provides information on the sample composition of the RNA species.

Ethidium bromide is an intercalating agent commonly used as a fluorescent tag in molecular biology laboratories for techniques such as agarose gel electrophoresis. It is commonly abbreviated as EtBr, which is also an abbreviation for bromoethane. To avoid confusion, some laboratories have used the abbreviation EthBr for this salt. When exposed to ultraviolet light, it will fluoresce with an orange colour, intensifying almost 20-fold after binding to DNA. Under the name homidium, it has been commonly used since the 1950s in veterinary medicine to treat trypanosomiasis in cattle. The high incidence of antimicrobial resistance makes this treatment impractical in some areas, where the related isometamidium chloride is used instead. Despite its reputation as a mutagen, tests have shown it to have low mutagenicity without metabolic activation.
HEPES is a zwitterionic sulfonic acid buffering agent; one of the twenty Good's buffers. HEPES is widely used in cell culture, largely because it is better at maintaining physiological pH despite changes in carbon dioxide concentration when compared to bicarbonate buffers, which are also commonly used in cell culture. Lepe-Zuniga et al. reported an unwanted photochemical process wherein HEPES catalyzes a reaction with riboflavin when exposed to ambient light to produce hydrogen peroxide. This is not a problem in bicarbonate-based cell culture buffers. It is therefore strongly advised to keep solutions containing both HEPES and riboflavin in darkness as much as possible to prevent oxidation.
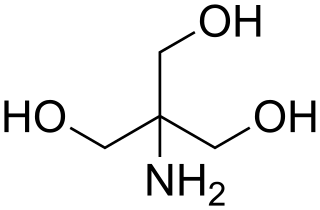
Tris, or tris(hydroxymethyl)aminomethane, or known during medical use as tromethamine or THAM, is an organic compound with the formula (HOCH2)3CNH2, one of the twenty Good's buffers. It is extensively used in biochemistry and molecular biology as a component of buffer solutions such as in TAE and TBE buffers, especially for solutions of nucleic acids. It contains a primary amine and thus undergoes the reactions associated with typical amines, e.g., condensations with aldehydes. Tris also complexes with metal ions in solution. In medicine, tromethamine is occasionally used as a drug, given in intensive care for its properties as a buffer for the treatment of severe metabolic acidosis in specific circumstances. Some medications are formulated as the "tromethamine salt" including Hemabate (carboprost as trometamol salt), and "ketorolac trometamol".
In biochemistry, isozymes are enzymes that differ in amino acid sequence but catalyze the same chemical reaction. Isozymes usually have different kinetic parameters, or are regulated differently. They permit the fine-tuning of metabolism to meet the particular needs of a given tissue or developmental stage.

Cathepsins are proteases found in all animals as well as other organisms. There are approximately a dozen members of this family, which are distinguished by their structure, catalytic mechanism, and which proteins they cleave. Most of the members become activated at the low pH found in lysosomes. Thus, the activity of this family lies almost entirely within those organelles. There are, however, exceptions such as cathepsin K, which works extracellularly after secretion by osteoclasts in bone resorption. Cathepsins have a vital role in mammalian cellular turnover.

Lithium acetate (CH3COOLi) is a salt of lithium and acetic acid. It is often abbreviated as LiOAc.
Good's buffers are twenty buffering agents for biochemical and biological research selected and described by Norman Good and colleagues during 1966–1980. Most of the buffers were new zwitterionic compounds prepared and tested by Good and coworkers for the first time, though some were known compounds previously overlooked by biologists. Before Good's work, few hydrogen ion buffers between pH 6 and 8 had been accessible to biologists, and very inappropriate, toxic, reactive and inefficient buffers had often been used. Many Good's buffers became and remain crucial tools in modern biological laboratories.

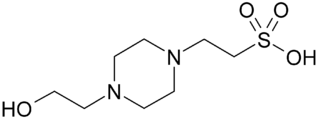
PIPES is the common name for piperazine-N,N′-bis(2-ethanesulfonic acid), and is a frequently used buffering agent in biochemistry. It is an ethanesulfonic acid buffer developed by Good et al. in the 1960s.
QPNC-PAGE, or Quantitative Preparative Native Continuous PolyAcrylamide Gel Electrophoresis, is a bioanalytical, one-dimensional, high-resolution and highly accurate technique applied in biochemistry and bioinorganic chemistry to separate proteins quantitatively by isoelectric point and by continuous elution from a gel column. This standardized variant of native gel electrophoresis and subset of preparative polyacrylamide gel electrophoresis is used by biologists to isolate macromolecules in solution, for example, active or native metalloproteins in biological samples or properly and improperly folded metal cofactor-containing proteins or protein isoforms in complex protein mixtures.

MES is the common name for the compound 2-(N-morpholino)ethanesulfonic acid. Its chemical structure contains a morpholine ring. It has a molecular weight of 195.2 and the chemical formula is C6H13NO4S. Synonyms include: 2-morpholinoethanesulfonic acid; 2-(4-morpholino)ethanesulfonic acid; 2-(N-morpholino)ethanesulfonic acid; 2-(4-morpholino)ethanesulfonic acid; MES; MES hydrate; and morpholine-4-ethanesulfonic acid hydrate. MOPS is a similar pH buffering compound which contains a propanesulfonic moiety instead of an ethanesulfonic one.

Klaus Weber was a German scientist who made many fundamentally important contributions to biochemistry, cell biology, and molecular biology, and was for many years the director of the Laboratory of Biochemistry and Cell Biology at the Max Planck Institute for Biophysical Chemistry in Göttingen, Germany.

Marmesin (nodakenetin) is a chemical compound precursor in psoralen and linear furanocoumarins biosynthesis.

MOPSO is a zwitterionic organic chemical buffering agent; one of Good's buffers. MOPSO and MOPS are chemically similar, differing only in the presence of a hydroxyl group on the C-2 of the propane moiety. It has a useful pH range of 6.5-7.9 in the physiological range, making it useful for cell culture work. It has a pKa of 6.9 with ΔpKa/°C of -0.015 and a solubility in water at 0°C of 0.75 M.
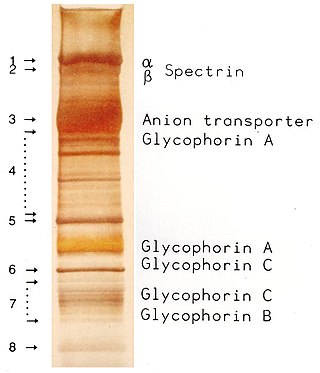
SDS-PAGE is a discontinuous electrophoretic system developed by Ulrich K. Laemmli which is commonly used as a method to separate proteins with molecular masses between 5 and 250 kDa. The combined use of sodium dodecyl sulfate and polyacrylamide gel eliminates the influence of structure and charge, and proteins are separated by differences in their size. At least up to 2012, the publication describing it was the most frequently cited paper by a single author, and the second most cited overall.

















