Related Research Articles

Argon is a chemical element; it has symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third most abundant gas in Earth's atmosphere, at 0.934%. It is more than twice as abundant as water vapor, 23 times as abundant as carbon dioxide, and more than 500 times as abundant as neon. Argon is the most abundant noble gas in Earth's crust, comprising 0.00015% of the crust.
A chemical element is a chemical substance that cannot be broken down into other substances by chemical reactions. The basic particle that constitutes a chemical element is the atom. Chemical elements are identified by the number of protons in the nuclei of their atoms, known as the element's atomic number. For example, oxygen has an atomic number of 8, meaning that each oxygen atom has 8 protons in its nucleus. Two or more atoms of the same element can combine to form molecules, in contrast to chemical compounds or mixtures, which contain atoms of different elements. Atoms can be transformed into different elements in nuclear reactions, which change an atom's atomic number.

The halogens are a group in the periodic table consisting of six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and the radioactive elements astatine (At) and tennessine (Ts), though some authors would exclude tennessine as its chemistry is unknown and is theoretically expected to be more like that of gallium. In the modern IUPAC nomenclature, this group is known as group 17.
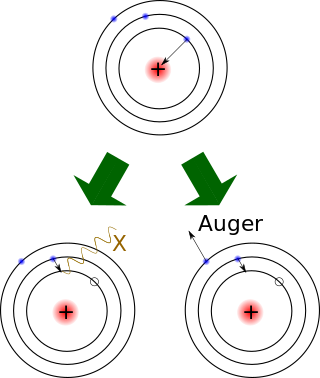
Electron capture is a process in which the proton-rich nucleus of an electrically neutral atom absorbs an inner atomic electron, usually from the K or L electron shells. This process thereby changes a nuclear proton to a neutron and simultaneously causes the emission of an electron neutrino.

In chemistry, the molar mass of a chemical compound is defined as the ratio between the mass and the amount of substance of any sample of the compound. The molar mass is a bulk, not molecular, property of a substance. The molar mass is an average of many instances of the compound, which often vary in mass due to the presence of isotopes. Most commonly, the molar mass is computed from the standard atomic weights and is thus a terrestrial average and a function of the relative abundance of the isotopes of the constituent atoms on Earth. The molar mass is appropriate for converting between the mass of a substance and the amount of a substance for bulk quantities.
Relative atomic mass, also known by the deprecated synonym atomic weight, is a dimensionless physical quantity defined as the ratio of the average mass of atoms of a chemical element in a given sample to the atomic mass constant. The atomic mass constant is defined as being 1/12 of the mass of a carbon-12 atom. Since both quantities in the ratio are masses, the resulting value is dimensionless. These definitions remain valid even after the 2019 redefinition of the SI base units.
Chemical species are a specific form of chemical substance or chemically identical molecular entities that have the same molecular energy level at a specified timescale. These entities are classified through bonding types and relative abundance of isotopes. Types of chemical species can be classified based on the type of molecular entity and can be either an atomic, molecular, ionic or radical species.

Positron emission, beta plus decay, or β+ decay is a subtype of radioactive decay called beta decay, in which a proton inside a radionuclide nucleus is converted into a neutron while releasing a positron and an electron neutrino. Positron emission is mediated by the weak force. The positron is a type of beta particle (β+), the other beta particle being the electron (β−) emitted from the β− decay of a nucleus.

The mass number (symbol A, from the German word: Atomgewicht, "atomic weight"), also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus. It is approximately equal to the atomic (also known as isotopic) mass of the atom expressed in atomic mass units. Since protons and neutrons are both baryons, the mass number A is identical with the baryon number B of the nucleus (and also of the whole atom or ion). The mass number is different for each isotope of a given chemical element, and the difference between the mass number and the atomic number Z gives the number of neutrons (N) in the nucleus: N = A − Z.

Raymond Davis Jr. was an American chemist and physicist. He is best known as the leader of the Homestake experiment in the 1960s-1980s, which was the first experiment to detect neutrinos emitted from the Sun; for this he shared the 2002 Nobel Prize in Physics.

A solar neutrino is a neutrino originating from nuclear fusion in the Sun's core, and is the most common type of neutrino passing through any source observed on Earth at any particular moment. Neutrinos are elementary particles with extremely small rest mass and a neutral electric charge. They only interact with matter via weak interaction and gravity, making their detection very difficult. This has led to the now-resolved solar neutrino problem. Much is now known about solar neutrinos, but research in this field is ongoing.
Natural palladium (46Pd) is composed of six stable isotopes, 102Pd, 104Pd, 105Pd, 106Pd, 108Pd, and 110Pd, although 102Pd and 110Pd are theoretically unstable. The most stable radioisotopes are 107Pd with a half-life of 6.5 million years, 103Pd with a half-life of 17 days, and 100Pd with a half-life of 3.63 days. Twenty-three other radioisotopes have been characterized with atomic weights ranging from 90.949 u (91Pd) to 128.96 u (129Pd). Most of these have half-lives that are less than a half an hour except 101Pd, 109Pd, and 112Pd.
Naturally occurring nickel (28Ni) is composed of five stable isotopes; 58
Ni
, 60
Ni
, 61
Ni
, 62
Ni
and 64
Ni
, with 58
Ni
being the most abundant. 26 radioisotopes have been characterised with the most stable being 59
Ni
with a half-life of 76,000 years, 63
Ni
with a half-life of 100.1 years, and 56
Ni
with a half-life of 6.077 days. All of the remaining radioactive isotopes have half-lives that are less than 60 hours and the majority of these have half-lives that are less than 30 seconds. This element also has 8 meta states.
Argon (18Ar) has 26 known isotopes, from 29Ar to 54Ar and 1 isomer (32mAr), of which three are stable. On the Earth, 40Ar makes up 99.6% of natural argon. The longest-lived radioactive isotopes are 39Ar with a half-life of 268 years, 42Ar with a half-life of 32.9 years, and 37Ar with a half-life of 35.04 days. All other isotopes have half-lives of less than two hours, and most less than one minute. The least stable is 29Ar with a half-life of approximately 4×10−20 seconds.
Chlorine (17Cl) has 25 isotopes, ranging from 28Cl to 52Cl, and two isomers, 34mCl and 38mCl. There are two stable isotopes, 35Cl (75.8%) and 37Cl (24.2%), giving chlorine a standard atomic weight of 35.45. The longest-lived radioactive isotope is 36Cl, which has a half-life of 301,000 years. All other isotopes have half-lives under 1 hour, many less than one second. The shortest-lived are proton-unbound 29Cl and 30Cl, with half-lives less than 10 picoseconds and 30 nanoseconds, respectively; the half-life of 28Cl is unknown.

A neutrino detector is a physics apparatus which is designed to study neutrinos. Because neutrinos only weakly interact with other particles of matter, neutrino detectors must be very large to detect a significant number of neutrinos. Neutrino detectors are often built underground, to isolate the detector from cosmic rays and other background radiation. The field of neutrino astronomy is still very much in its infancy – the only confirmed extraterrestrial sources as of 2018 are the Sun and the supernova 1987A in the nearby Large Magellanic Cloud. Another likely source is the blazar TXS 0506+056 about 3.7 billion light years away. Neutrino observatories will "give astronomers fresh eyes with which to study the universe".

The Homestake experiment was an experiment headed by astrophysicists Raymond Davis, Jr. and John N. Bahcall in the late 1960s. Its purpose was to collect and count neutrinos emitted by nuclear fusion taking place in the Sun. Bahcall performed the theoretical calculations and Davis designed the experiment. After Bahcall calculated the rate at which the detector should capture neutrinos, Davis's experiment turned up only one third of this figure. The experiment was the first to successfully detect and count solar neutrinos, and the discrepancy in results created the solar neutrino problem. The experiment operated continuously from 1970 until 1994. The University of Pennsylvania took it over in 1984. The discrepancy between the predicted and measured rates of neutrino detection was later found to be due to neutrino "flavour" oscillations.
The standard solar model (SSM) is a mathematical treatment of the Sun as a spherical ball of gas. This model, technically the spherically symmetric quasi-static model of a star, has stellar structure described by several differential equations derived from basic physical principles. The model is constrained by boundary conditions, namely the luminosity, radius, age and composition of the Sun, which are well determined. The age of the Sun cannot be measured directly; one way to estimate it is from the age of the oldest meteorites, and models of the evolution of the Solar System. The composition in the photosphere of the modern-day Sun, by mass, is 74.9% hydrogen and 23.8% helium. All heavier elements, called metals in astronomy, account for less than 2 percent of the mass. The SSM is used to test the validity of stellar evolution theory. In fact, the only way to determine the two free parameters of the stellar evolution model, the helium abundance and the mixing length parameter, are to adjust the SSM to "fit" the observed Sun.

The standard atomic weight of a chemical element (symbol Ar°(E) for element "E") is the weighted arithmetic mean of the relative isotopic masses of all isotopes of that element weighted by each isotope's abundance on Earth. For example, isotope 63Cu (Ar = 62.929) constitutes 69% of the copper on Earth, the rest being 65Cu (Ar = 64.927), so
GALLEX or Gallium Experiment was a radiochemical neutrino detection experiment that ran between 1991 and 1997 at the Laboratori Nazionali del Gran Sasso (LNGS). This project was performed by an international collaboration of French, German, Italian, Israeli, Polish and American scientists led by the Max-Planck-Institut für Kernphysik Heidelberg. After brief interruption, the experiment was continued under a new name GNO from May 1998 to April 2003.
References
- ↑ Wieser, M. E. (2006), "Atomic Weights of the Elements 2005" (PDF), Pure and Applied Chemistry , 78 (11): 2051–66, doi: 10.1351/pac200678112051
- 1 2 J.N. Bahcall (1969). "Neutrinos from the Sun". Scientific American . 221 (1): 28–37. Bibcode:1969SciAm.221a..28B. doi:10.1038/scientificamerican0769-28.
- 1 2 3 Sutton, Christine (1992). Spaceship Neutrino . Cambridge University Press. pp. 151–152. ISBN 978-0-521-36404-1. OCLC 25246163.
chlorine-37 neutrino.
- ↑ F.H. Shu (1982). The Physical Universe: An Introduction to Astronomy . University Science Books. p. 122. ISBN 978-0-935702-05-7.
chlorine 37 neutrino.
- ↑ A.H. Snell, F. Pleasonton (1955). "Spectrometry of the Neutrino Recoils of Argon-37". Physical Review . 100 (5): 1396–1403. Bibcode:1955PhRv..100.1396S. doi:10.1103/PhysRev.100.1396.
- ↑ A. Bhatnagar, W. Livingston (2005). Fundamental of Solar Astronomy. World Scientific. pp. 87–89. ISBN 978-981-238-244-3.
- ↑ Rosman, K. J. R.; Taylor, P. D. P. (1998), "Isotopic Compositions of the Elements 1997" (PDF), Pure and Applied Chemistry , 70 (1): 217–35, doi:10.1351/pac199870010217
- 1 2 de Laeter, J. R.; et al. (2003), "Atomic Weights of the Elements: Review 2000", Pure and Applied Chemistry , 75 (6): 683–800, doi: 10.1351/pac200375060683