
G protein-coupled receptors (GPCRs), also known as seven-(pass)-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptors, and G protein-linked receptors (GPLR), form a large group of evolutionarily related proteins that are cell surface receptors that detect molecules outside the cell and activate cellular responses. Since they are coupled with G proteins, they pass through the cell membrane seven times in form of six loops of amino acid residues, which is why they are sometimes referred to as seven-transmembrane receptors. Ligands can bind either to the extracellular N-terminus and loops or to the binding site within transmembrane helices. They are all activated by agonists, although a spontaneous auto-activation of an empty receptor has also been observed.

Structural biology is a field that is many centuries old which, as defined by the Journal of Structural Biology, deals with structural analysis of living material at every level of organization. Early structural biologists throughout the 19th and early 20th centuries were primarily only able to study structures to the limit of the naked eye's visual acuity and through magnifying glasses and light microscopes.
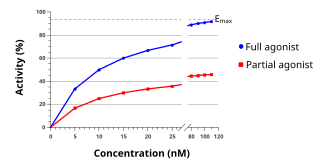
An agonist is a chemical that activates a receptor to produce a biological response. Receptors are cellular proteins whose activation causes the cell to modify what it is currently doing. In contrast, an antagonist blocks the action of the agonist, while an inverse agonist causes an action opposite to that of the agonist.
Functional selectivity is the ligand-dependent selectivity for certain signal transduction pathways relative to a reference ligand at the same receptor. Functional selectivity can be present when a receptor has several possible signal transduction pathways. To which degree each pathway is activated thus depends on which ligand binds to the receptor. Functional selectivity, or biased signaling, is most extensively characterized at G protein coupled receptors (GPCRs). A number of biased agonists, such as those at muscarinic M2 receptors tested as analgesics or antiproliferative drugs, or those at opioid receptors that mediate pain, show potential at various receptor families to increase beneficial properties while reducing side effects. For example, pre-clinical studies with G protein biased agonists at the μ-opioid receptor show equivalent efficacy for treating pain with reduced risk for addictive potential and respiratory depression. Studies within the chemokine receptor system also suggest that GPCR biased agonism is physiologically relevant. For example, a beta-arrestin biased agonist of the chemokine receptor CXCR3 induced greater chemotaxis of T cells relative to a G protein biased agonist.
GABAB receptors (GABABR) are G-protein coupled receptors for gamma-aminobutyric acid (GABA), therefore making them metabotropic receptors, that are linked via G-proteins to potassium channels. The changing potassium concentrations hyperpolarize the cell at the end of an action potential. The reversal potential of the GABAB-mediated IPSP is –100 mV, which is much more hyperpolarized than the GABAA IPSP. GABAB receptors are found in the central nervous system and the autonomic division of the peripheral nervous system.
A receptor activated solely by a synthetic ligand (RASSL) or designer receptor exclusively activated by designer drugs (DREADD), is a class of artificially engineered protein receptors used in the field of chemogenetics which are selectively activated by certain ligands. They are used in biomedical research, in particular in neuroscience to manipulate the activity of neurons.
The beta-2 adrenergic receptor, also known as ADRB2, is a cell membrane-spanning beta-adrenergic receptor that binds epinephrine (adrenaline), a hormone and neurotransmitter whose signaling, via adenylate cyclase stimulation through trimeric Gs proteins, increased cAMP, and downstream L-type calcium channel interaction, mediates physiologic responses such as smooth muscle relaxation and bronchodilation.

Arrestins are a small family of proteins important for regulating signal transduction at G protein-coupled receptors. Arrestins were first discovered as a part of a conserved two-step mechanism for regulating the activity of G protein-coupled receptors (GPCRs) in the visual rhodopsin system by Hermann Kühn, Scott Hall, and Ursula Wilden and in the β-adrenergic system by Martin J. Lohse and co-workers.

Richard Henderson is a British molecular biologist and biophysicist and pioneer in the field of electron microscopy of biological molecules. Henderson shared the Nobel Prize in Chemistry in 2017 with Jacques Dubochet and Joachim Frank.

The nociceptin opioid peptide receptor (NOP), also known as the nociceptin/orphanin FQ (N/OFQ) receptor or kappa-type 3 opioid receptor, is a protein that in humans is encoded by the OPRL1 gene. The nociceptin receptor is a member of the opioid subfamily of G protein-coupled receptors whose natural ligand is the 17 amino acid neuropeptide known as nociceptin (N/OFQ). This receptor is involved in the regulation of numerous brain activities, particularly instinctive and emotional behaviors. Antagonists targeting NOP are under investigation for their role as treatments for depression and Parkinson's disease, whereas NOP agonists have been shown to act as powerful, non-addictive painkillers in non-human primates.

The alpha-2B adrenergic receptor, is a G-protein coupled receptor. It is a subtype of the adrenergic receptor family. The human gene encoding this receptor has the symbol ADRA2B. ADRA2B orthologs have been identified in several mammals.

Arrestin, beta 1, also known as ARRB1, is a protein which in humans is encoded by the ARRB1 gene.

G protein-coupled receptor kinase 5 is a member of the G protein-coupled receptor kinase subfamily of the Ser/Thr protein kinases, and is most highly similar to GRK4 and GRK6. The protein phosphorylates the activated forms of G protein-coupled receptors to regulate their signaling.

Rauwolscine, also known as isoyohimbine, α-yohimbine, and corynanthidine, is an alkaloid found in various species within the genera Rauvolfia and Corynanthe. It is a stereoisomer of yohimbine. Rauwolscine is a central nervous system stimulant, a local anesthetic and a vague aphrodisiac.

Brian Kent Kobilka is an American physiologist and a recipient of the 2012 Nobel Prize in Chemistry with Robert Lefkowitz for discoveries that reveal the workings of G protein-coupled receptors. He is currently a professor in the department of Molecular and Cellular Physiology at Stanford University School of Medicine. He is also a co-founder of ConfometRx, a biotechnology company focusing on G protein-coupled receptors. He was named a member of the National Academy of Sciences in 2011.

David Jay Julius is an American physiologist and Nobel Prize laureate known for his work on molecular mechanisms of pain sensation and heat, including the characterization of the TRPV1 and TRPM8 receptors that detect capsaicin, menthol, and temperature. He is a professor at the University of California, San Francisco.

A GPCR oligomer is a protein complex that consists of a small number of G protein-coupled receptors (GPCRs). It is held together by covalent bonds or by intermolecular forces. The subunits within this complex are called protomers, while unconnected receptors are called monomers. Receptor homomers consist of identical protomers, while heteromers consist of different protomers.

PZM21 is an experimental opioid analgesic drug that is being researched for the treatment of pain. It is claimed to be a functionally selective μ-opioid receptor agonist which produces μ-opioid receptor mediated G protein signaling, with potency and efficacy similar to morphine, but with less β-arrestin 2 recruitment. However, recent reports highlight that this might be due to its low intrinsic efficacy, rather than functional selectivity or 'G protein bias' as initially reported. In tests on mice, PZM21 was slightly less potent than morphine or TRV130 as an analgesic, but also had significantly reduced adverse effects, with less constipation than morphine, and very little respiratory depression, even at high doses. This research was described as a compelling example of how modern high-throughput screening techniques can be used to discover new chemotypes with specific activity profiles, even at targets such as the μ-opioid receptor which have already been thoroughly investigated. More recent research has suggested however that at higher doses, PZM21 is capable of producing classic opioid side effects such as respiratory depression and development of tolerance and may have only limited functional selectivity.

Kiyoshi Nagai was a Japanese structural biologist at the MRC Laboratory of Molecular Biology Cambridge, UK. He was known for his work on the mechanism of RNA splicing and structures of the spliceosome.

Jan Steyaert is a Belgian bioengineer and molecular biologist. He started his career as an enzymologist but the Steyaertlab is best known for pioneering work on (engineered) nanobodies for applications in structural biology, omics and drug design. He is full professor and teaches biochemistry at the Vrije Universiteit Brussel and Director of the VIB-VUB Center for Structural Biology, one of the Research Centers of the Vlaams Instituut voor Biotechnologie (VIB). He was involved in the foundation of three spin-off companies: Ablynx, Biotalys, and Confo Therapeutics.

















