The dalton or unified atomic mass unit is a non-SI unit of mass defined as 1/12 of the mass of an unbound neutral atom of carbon-12 in its nuclear and electronic ground state and at rest. The atomic mass constant, denoted mu, is defined identically, giving mu = 1/12 m(12C) = 1 Da.
Relative atomic mass, also known by the deprecated synonym atomic weight, is a dimensionless physical quantity defined as the ratio of the average mass of atoms of a chemical element in a given sample to the atomic mass constant. The atomic mass constant is defined as being 1/12 of the mass of a carbon-12 atom. Since both quantities in the ratio are masses, the resulting value is dimensionless. These definitions remain valid even after the 2019 redefinition of the SI base units.
In chemistry, the amount of substance (symbol n) in a given sample of matter is defined as a ratio (n = N/NA) between the number of elementary entities (N) and the Avogadro constant (NA). The entities are usually molecules, atoms, or ions of a specified kind. The particular substance sampled may be specified using a subscript, e.g., the amount of sodium chloride (NaCl) would be denoted as nNaCl. The unit of amount of substance in the International System of Units is the mole (symbol: mol), a base unit. Since 2019, the value of the Avogadro constant NA is defined to be exactly 6.02214076×1023 mol−1. Sometimes, the amount of substance is referred to as the chemical amount or, informally, as the "number of moles" in a given sample of matter.
Vienna Standard Mean Ocean Water (VSMOW) is an isotopic standard for water, that is, a particular sample of water whose proportions of different isotopes of hydrogen and oxygen are accurately known. VSMOW is distilled from ocean water and does not contain salt or other impurities. Published and distributed by the Vienna-based International Atomic Energy Agency in 1968, the standard and its essentially identical successor, VSMOW2, continue to be used as a reference material.
Naturally occurring erbium (68Er) is composed of 6 stable isotopes, with 166Er being the most abundant. 39 radioisotopes have been characterized with between 74 and 112 neutrons, or 142 to 180 nucleons, with the most stable being 169Er with a half-life of 9.4 days, 172Er with a half-life of 49.3 hours, 160Er with a half-life of 28.58 hours, 165Er with a half-life of 10.36 hours, and 171Er with a half-life of 7.516 hours. All of the remaining radioactive isotopes have half-lives that are less than 3.5 hours, and the majority of these have half-lives that are less than 4 minutes. This element also has numerous meta states, with the most stable being 167mEr.
Natural holmium (67Ho) contains one observationally stable isotope, 165Ho. The below table lists 36 isotopes spanning 140Ho through 175Ho as well as 33 nuclear isomers. Among the known synthetic radioactive isotopes; the most stable one is 163Ho, with a half-life of 4,570 years. All other radioisotopes have half-lives not greater than 1.117 days in their ground states, and most have half-lives under 3 hours.
Naturally occurring terbium (65Tb) is composed of one stable isotope, 159Tb. Thirty-seven radioisotopes have been characterized, with the most stable being 158Tb with a half-life of 180 years, 157Tb with a half-life of 71 years, and 160Tb with a half-life of 72.3 days. All of the remaining radioactive isotopes have half-lives that are less than 6.907 days, and the majority of these have half-lives that are less than 24 seconds. This element also has 27 meta states, with the most stable being 156m1Tb, 154m2Tb and 154m1Tb.
Naturally occurring praseodymium (59Pr) is composed of one stable isotope, 141Pr. Thirty-eight radioisotopes have been characterized with the most stable being 143Pr, with a half-life of 13.57 days and 142Pr, with a half-life of 19.12 hours. All of the remaining radioactive isotopes have half-lives that are less than 5.985 hours and the majority of these have half-lives that are less than 33 seconds. This element also has 15 meta states with the most stable being 138mPr, 142mPr and 134mPr.
Antimony (51Sb) occurs in two stable isotopes, 121Sb and 123Sb. There are 35 artificial radioactive isotopes, the longest-lived of which are 125Sb, with a half-life of 2.75856 years; 124Sb, with a half-life of 60.2 days; and 126Sb, with a half-life of 12.35 days. All other isotopes have half-lives less than 4 days, most less than an hour.
Indium (49In) consists of two primordial nuclides, with the most common (~ 95.7%) nuclide (115In) being measurably though weakly radioactive. Its spin-forbidden decay has a half-life of 4.41×1014 years, much longer than the currently accepted age of the Universe.
Naturally occurring rhodium (45Rh) is composed of only one stable isotope, 103Rh. The most stable radioisotopes are 101Rh with a half-life of 3.3 years, 102Rh with a half-life of 207 days, and 99Rh with a half-life of 16.1 days. Thirty other radioisotopes have been characterized with atomic weights ranging from 88.949 u (89Rh) to 121.943 u (122Rh). Most of these have half-lives that are less than an hour except 100Rh and 105Rh. There are also numerous meta states with the most stable being 102mRh (0.141 MeV) with a half-life of about 3.7 years and 101mRh (0.157 MeV) with a half-life of 4.34 days.
Natural yttrium (39Y) is composed of a single isotope yttrium-89. The most stable radioisotopes are 88Y, which has a half-life of 106.6 days and 91Y with a half-life of 58.51 days. All the other isotopes have half-lives of less than a day, except 87Y, which has a half-life of 79.8 hours, and 90Y, with 64 hours. The dominant decay mode below the stable 89Y is electron capture and the dominant mode after it is beta emission. Thirty-five unstable isotopes have been characterized.
Arsenic (33As) has 33 known isotopes and at least 10 isomers. Only one of these isotopes, 75As, is stable; as such, it is considered a monoisotopic element. The longest-lived radioisotope is 73As with a half-life of 80 days. Arsenic has been proposed as a "salting" material for nuclear weapons. A jacket of 75As, irradiated by the intense high-energy neutron flux from an exploding thermonuclear weapon, would transmute into the radioactive isotope 76As with a half-life of 1.0778 days and produce approximately 1.13 MeV gamma radiation, significantly increasing the radioactivity of the weapon's fallout for several hours. Such a weapon is not known to have ever been built, tested, or used.
Naturally occurring zinc (30Zn) is composed of the 5 stable isotopes 64Zn, 66Zn, 67Zn, 68Zn, and 70Zn with 64Zn being the most abundant. Twenty-five radioisotopes have been characterised with the most abundant and stable being 65Zn with a half-life of 244.26 days, and 72Zn with a half-life of 46.5 hours. All of the remaining radioactive isotopes have half-lives that are less than 14 hours and the majority of these have half-lives that are less than 1 second. This element also has 10 meta states.
Naturally occurring cobalt (27Co) consists of a single stable isotope, 59Co. Twenty-eight radioisotopes have been characterized; the most stable are 60Co with a half-life of 5.2714 years, 57Co, 56Co, and 58Co. All other isotopes have half-lives of less than 18 hours and most of these have half-lives of less than 1 second. This element also has 11 meta states, all of which have half-lives of less than 15 minutes.
Naturally occurring chromium (24Cr) is composed of four stable isotopes; 50Cr, 52Cr, 53Cr, and 54Cr with 52Cr being the most abundant (83.789% natural abundance). 50Cr is suspected of decaying by β+β+ to 50Ti with a half-life of (more than) 1.8×1017 years. Twenty-two radioisotopes, all of which are entirely synthetic, have been characterized, the most stable being 51Cr with a half-life of 27.7 days. All of the remaining radioactive isotopes have half-lives that are less than 24 hours and the majority of these have half-lives that are less than 1 minute. This element also has two meta states, 45mCr, the more stable one, and 59mCr, the least stable isotope or isomer.
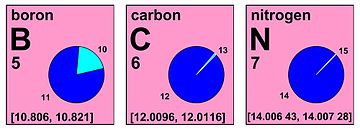
Natural nitrogen (7N) consists of two stable isotopes: the vast majority (99.6%) of naturally occurring nitrogen is nitrogen-14, with the remainder being nitrogen-15. Thirteen radioisotopes are also known, with atomic masses ranging from 9 to 23, along with three nuclear isomers. All of these radioisotopes are short-lived, the longest-lived being nitrogen-13 with a half-life of 9.965(4) min. All of the others have half-lives below 7.15 seconds, with most of these being below 620 milliseconds. Most of the isotopes with atomic mass numbers below 14 decay to isotopes of carbon, while most of the isotopes with masses above 15 decay to isotopes of oxygen. The shortest-lived known isotope is nitrogen-10, with a half-life of 143(36) yoctoseconds, though the half-life of nitrogen-9 has not been measured exactly.

The standard atomic weight of a chemical element (symbol Ar°(E) for element "E") is the weighted arithmetic mean of the relative isotopic masses of all isotopes of that element weighted by each isotope's abundance on Earth. For example, isotope 63Cu (Ar = 62.929) constitutes 69% of the copper on Earth, the rest being 65Cu (Ar = 64.927), so

The mass recorded by a mass spectrometer can refer to different physical quantities depending on the characteristics of the instrument and the manner in which the mass spectrum is displayed.
The Inorganic Chemistry Division of the International Union of Pure and Applied Chemistry (IUPAC), also known as Division II, deals with all aspects of inorganic chemistry, including materials and bioinorganic chemistry, and also with isotopes, atomic weights and the periodic table. It furthermore advises the Chemical Nomenclature and Structure Representation Division on issues dealing with inorganic compounds and materials. For the general public, the most visible result of the division's work is that it evaluates and advises the IUPAC on names and symbols proposed for new elements that have been approved for addition to the periodic table. For the scientific end educational community the work on isotopic abundances and atomic weights is of fundamental importance as these numbers are continuously checked and updated.