Related Research Articles

Microscopy is the technical field of using microscopes to view objects and areas of objects that cannot be seen with the naked eye. There are three well-known branches of microscopy: optical, electron, and scanning probe microscopy, along with the emerging field of X-ray microscopy.

The optical microscope, also referred to as a light microscope, is a type of microscope that commonly uses visible light and a system of lenses to generate magnified images of small objects. Optical microscopes are the oldest design of microscope and were possibly invented in their present compound form in the 17th century. Basic optical microscopes can be very simple, although many complex designs aim to improve resolution and sample contrast.

In mathematics, deconvolution is an algorithm-based process used to enhance signals from recorded data. Where the recorded data can be modeled as a pure signal that is distorted by a filter, deconvolution can be used to restore the original signal. The concept of deconvolution is widely used in the techniques of signal processing and image processing.

Computational photography refers to digital image capture and processing techniques that use digital computation instead of optical processes. Computational photography can improve the capabilities of a camera, or introduce features that were not possible at all with film based photography, or reduce the cost or size of camera elements. Examples of computational photography include in-camera computation of digital panoramas, high-dynamic-range images, and light field cameras. Light field cameras use novel optical elements to capture three dimensional scene information which can then be used to produce 3D images, enhanced depth-of-field, and selective de-focusing. Enhanced depth-of-field reduces the need for mechanical focusing systems. All of these features use computational imaging techniques.
Super-resolution imaging (SR) is a class of techniques that enhance (increase) the resolution of an imaging system. In optical SR the diffraction limit of systems is transcended, while in geometrical SR the resolution of digital imaging sensors is enhanced.
Nanophotonics or nano-optics is the study of the behavior of light on the nanometer scale, and of the interaction of nanometer-scale objects with light. It is a branch of optics, optical engineering, electrical engineering, and nanotechnology. It often involves metallic components, which can transport and focus light via surface plasmon polaritons.
Digital holography refers to the acquisition and processing of holograms with a digital sensor array , typically a CCD camera or a similar device. Image rendering, or reconstruction of object data is performed numerically from digitized interferograms. Digital holography offers a means of measuring optical phase data and typically delivers three-dimensional surface or optical thickness images. Several recording and processing schemes have been developed to assess optical wave characteristics such as amplitude, phase, and polarization state, which make digital holography a very powerful method for metrology applications .
Computer-generated holography (CGH) is the method of digitally generating holographic interference patterns. A holographic image can be generated e.g. by digitally computing a holographic interference pattern and printing it onto a mask or film for subsequent illumination by suitable coherent light source.

Second-harmonic imaging microscopy (SHIM) is based on a nonlinear optical effect known as second-harmonic generation (SHG). SHIM has been established as a viable microscope imaging contrast mechanism for visualization of cell and tissue structure and function. A second-harmonic microscope obtains contrasts from variations in a specimen's ability to generate second-harmonic light from the incident light while a conventional optical microscope obtains its contrast by detecting variations in optical density, path length, or refractive index of the specimen. SHG requires intense laser light passing through a material with a noncentrosymmetric molecular structure. Second-harmonic light emerging from an SHG material is exactly half the wavelength (frequency doubled) of the light entering the material. While two-photon-excited fluorescence (TPEF) is also a two photon process, TPEF loses some energy during the relaxation of the excited state, while SHG is energy conserving. Typically, an inorganic crystal is used to produce SHG light such as lithium niobate (LiNbO3), potassium titanyl phosphate (KTP = KTiOPO4), and lithium triborate (LBO = LiB3O5). Though SHG requires a material to have specific molecular orientation in order for the incident light to be frequency doubled, some biological materials can be highly polarizable, and assemble into fairly ordered, large noncentrosymmetric structures. Biological materials such as collagen, microtubules, and muscle myosin can produce SHG signals. The SHG pattern is mainly determined by the phase matching condition. A common setup for an SHG imaging system will have a laser scanning microscope with a titanium sapphire mode-locked laser as the excitation source. The SHG signal is propagated in the forward direction. However, some experiments have shown that objects on the order of about a tenth of the wavelength of the SHG produced signal will produce nearly equal forward and backward signals.
The time-stretch analog-to-digital converter (TS-ADC), also known as the time-stretch enhanced recorder (TiSER), is an analog-to-digital converter (ADC) system that has the capability of digitizing very high bandwidth signals that cannot be captured by conventional electronic ADCs. Alternatively, it is also known as the photonic time-stretch (PTS) digitizer, since it uses an optical frontend. It relies on the process of time-stretch, which effectively slows down the analog signal in time before it can be digitized by a standard electronic ADC.
Super-resolution microscopy is a series of techniques in optical microscopy that allow such images to have resolutions higher than those imposed by the diffraction limit, which is due to the diffraction of light. Super-resolution imaging techniques rely on the near-field or on the far-field. Among techniques that rely on the latter are those that improve the resolution only modestly beyond the diffraction-limit, such as confocal microscopy with closed pinhole or aided by computational methods such as deconvolution or detector-based pixel reassignment, the 4Pi microscope, and structured-illumination microscopy technologies such as SIM and SMI.
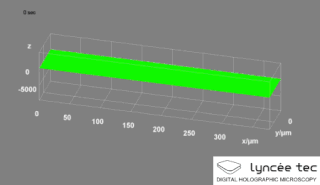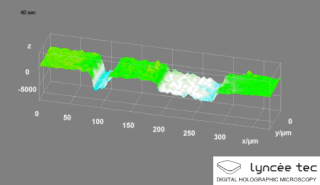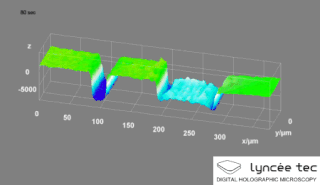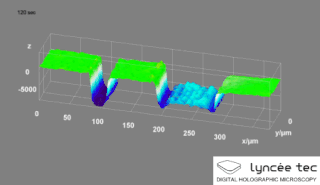
Digital holographic microscopy (DHM) is digital holography applied to microscopy. Digital holographic microscopy distinguishes itself from other microscopy methods by not recording the projected image of the object. Instead, the light wave front information originating from the object is digitally recorded as a hologram, from which a computer calculates the object image by using a numerical reconstruction algorithm. The image forming lens in traditional microscopy is thus replaced by a computer algorithm. Other closely related microscopy methods to digital holographic microscopy are interferometric microscopy, optical coherence tomography and diffraction phase microscopy. Common to all methods is the use of a reference wave front to obtain amplitude (intensity) and phase information. The information is recorded on a digital image sensor or by a photodetector from which an image of the object is created (reconstructed) by a computer. In traditional microscopy, which do not use a reference wave front, only intensity information is recorded and essential information about the object is lost.
Serial time-encoded amplified imaging/microscopy or stretched time-encoded amplified imaging/microscopy' (STEAM) is a fast real-time optical imaging method that provides MHz frame rate, ~100 ps shutter speed, and ~30 dB optical image gain. Based on the Photonic Time Stretch technique, STEAM holds world records for shutter speed and frame rate in continuous real-time imaging. STEAM employs the Photonic Time Stretch with internal Raman amplification to realize optical image amplification to circumvent the fundamental trade-off between sensitivity and speed that affects virtually all optical imaging and sensing systems. This method uses a single-pixel photodetector, eliminating the need for the detector array and readout time limitations. Avoiding this problem and featuring the optical image amplification for dramatic improvement in sensitivity at high image acquisition rates, STEAM's shutter speed is at least 1000 times faster than the state-of-the-art CCD and CMOS cameras. Its frame rate is 1000 times faster than fastest CCD cameras and 10-100 times faster than fastest CMOS cameras.
Endomicroscopy is a technique for obtaining histology-like images from inside the human body in real-time, a process known as ‘optical biopsy’. It generally refers to fluorescence confocal microscopy, although multi-photon microscopy and optical coherence tomography have also been adapted for endoscopic use. Commercially available clinical and pre-clinical endomicroscopes can achieve a resolution on the order of a micrometre, have a field-of-view of several hundred µm, and are compatible with fluorophores which are excitable using 488 nm laser light. The main clinical applications are currently in imaging of the tumour margins of the brain and gastro-intestinal tract, particularly for the diagnosis and characterisation of Barrett’s Esophagus, pancreatic cysts and colorectal lesions. A number of pre-clinical and transnational applications have been developed for endomicroscopy as it enables researchers to perform live animal imaging. Major pre-clinical applications are in gastro-intestinal tract, toumous margin detection, uterine complications, ischaemia, live imaging of cartilage and tendon, organoid imaging etc.

Quantitative phase contrast microscopy or quantitative phase imaging are the collective names for a group of microscopy methods that quantify the phase shift that occurs when light waves pass through a more optically dense object.
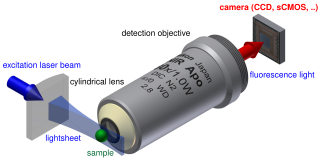
Light sheet fluorescence microscopy (LSFM) is a fluorescence microscopy technique with an intermediate-to-high optical resolution, but good optical sectioning capabilities and high speed. In contrast to epifluorescence microscopy only a thin slice of the sample is illuminated perpendicularly to the direction of observation. For illumination, a laser light-sheet is used, i.e. a laser beam which is focused only in one direction. A second method uses a circular beam scanned in one direction to create the lightsheet. As only the actually observed section is illuminated, this method reduces the photodamage and stress induced on a living sample. Also the good optical sectioning capability reduces the background signal and thus creates images with higher contrast, comparable to confocal microscopy. Because LSFM scans samples by using a plane of light instead of a point, it can acquire images at speeds 100 to 1000 times faster than those offered by point-scanning methods.

Fourier ptychography is a computational imaging technique based on optical microscopy that consists in the synthesis of a wider numerical aperture from a set of full-field images acquired at various coherent illumination angles, resulting in increased resolution compared to a conventional microscope.
Computational Imaging is the process of indirectly forming images from measurements using algorithms that rely on a significant amount of computing. In contrast to traditional imaging, computational imaging systems involve a tight integration of the sensing system and the computation in order to form the images of interest. The ubiquitous availability of fast computing platforms, the advances in algorithms and modern sensing hardware is resulting in imaging systems with significantly enhanced capabilities. Computational Imaging systems cover a broad range of applications include computational microscopy, tomographic imaging, MRI, ultrasound imaging, computational photography, Synthetic Aperture Radar (SAR), seismic imaging etc. The integration of the sensing and the computation in computational imaging systems allows for accessing information which was otherwise not possible. For example:

Laura Ann Waller is a computer scientist and Ted Van Duzer Endowed Associate Professor at the University of California, Berkeley. She was awarded a Chan Zuckerberg Initiative Fellowship to develop microscopes to image deep structures within the brain in 2017 and won the 2018 SPIE Early Career Award.
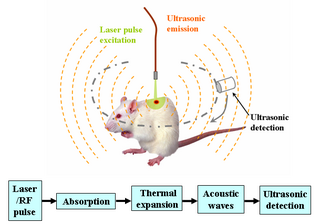
Deep learning in photoacoustic imaging combines the hybrid imaging modality of photoacoustic imaging (PA) with the rapidly evolving field of deep learning. Photoacoustic imaging is based on the photoacoustic effect, in which optical absorption causes a rise in temperature, which causes a subsequent rise in pressure via thermo-elastic expansion. This pressure rise propagates through the tissue and is sensed via ultrasonic transducers. Due to the proportionality between the optical absorption, the rise in temperature, and the rise in pressure, the ultrasound pressure wave signal can be used to quantify the original optical energy deposition within the tissue.
References
- ↑ Ikoma, Hayato. "Computational microscopy for sample analysis." PhD diss., Massachusetts Institute of Technology, 2014.
- ↑ de Haan, Kevin, Yair Rivenson, Yichen Wu, and Aydogan Ozcan. "Deep-learning-based image reconstruction and enhancement in optical microscopy." Proceedings of the IEEE 108, no. 1 (2019): 30-50.
- ↑ Waller, Laura, and Lei Tian. "Computational imaging: Machine learning for 3D microscopy." Nature 523.7561 (2015): 416-417.
- ↑ Yeh, Li-Hao, Shwetadwip Chowdhury, Nicole A. Repina, and Laura Waller. "Speckle-structured illumination for 3D phase and fluorescence computational microscopy." Biomedical optics express 10, no. 7 (2019): 3635-3653.
- ↑ McLeod, Euan, and Aydogan Ozcan. "Unconventional methods of imaging: computational microscopy and compact implementations." Reports on Progress in Physics 79, no. 7 (2016): 076001.
- ↑ Horstmeyer, Roarke. Computational microscopy: Turning megapixels into gigapixels. 2016.
- ↑ Pham, Minh. "New Algorithms in Computational Microscopy." PhD diss., UCLA, 2020.
- ↑ Chen, Claire Lifan, Ata Mahjoubfar, Li-Chia Tai, Ian K. Blaby, Allen Huang, Kayvan Reza Niazi, and Bahram Jalali. "Deep learning in label-free cell classification." Scientific Reports 6 (2016): 21471.
- ↑ Yuan, Shuai, and Chrysanthe Preza. "Point-spread function engineering to reduce the impact of spherical aberration on 3D computational fluorescence microscopy imaging." Optics Express 19, no. 23 (2011): 23298-23314.
- ↑ Hollmann, Joseph L., Andrew K. Dunn, and Charles A. DiMarzio. "Computational microscopy in embryo imaging." Optics Letters 29, no. 19 (2004): 2267-2269.
- ↑ Eggeling, Christian, and Alf Honigmann. "Closing the gap: the approach of optical and computational microscopy to uncover biomembrane organization." Biochimica et Biophysica Acta (BBA)-Biomembranes 1858, no. 10 (2016): 2558-2568.
- ↑ Greenbaum, Alon, Yibo Zhang, Alborz Feizi, Ping-Luen Chung, Wei Luo, Shivani R. Kandukuri, and Aydogan Ozcan. "Wide-field computational imaging of pathology slides using lens-free on-chip microscopy." Science translational medicine 6, no. 267 (2014): 267ra175-267ra175.