
Saffron is a spice derived from the flower of Crocus sativus, commonly known as the "saffron crocus". The vivid crimson stigma and styles, called threads, are collected and dried for use mainly as a seasoning and colouring agent in food. Although some doubts remain on its origin, it is believed that saffron originated in Iran. However, Greece and Mesopotamia have also been suggested as the possible region of origin of this plant. The saffron crocus slowly propagated throughout much of Eurasia and was later brought to parts of North Africa, North America, and Oceania.

Crocus is a genus of seasonal flowering plants in the family Iridaceae comprising about 100 species of perennials growing from corms. They are low growing plants, whose flower stems remain underground, that bear relatively large white, yellow, orange or purple flowers and then become dormant after flowering. Many are cultivated for their flowers, appearing in autumn, winter, or spring. The flowers close at night and in overcast weather conditions. The crocus has been known throughout recorded history, mainly as the source of saffron. Saffron is obtained from the dried stigma of Crocus sativus, an autumn-blooming species. It is valued as a spice and dyestuff, and is one of the most expensive spices in the world. Iran is the center of saffron production. Crocuses are native to woodland, scrub, and meadows from sea level to alpine tundra from the Mediterranean, through North Africa, central and southern Europe, the islands of the Aegean, the Middle East and across Central Asia to Xinjiang in western China. Crocuses may be propagated from seed or from daughter cormels formed on the corm, that eventually produce mature plants. They arrived in Europe from Turkey in the 16th century and became valued as an ornamental flowering plant.
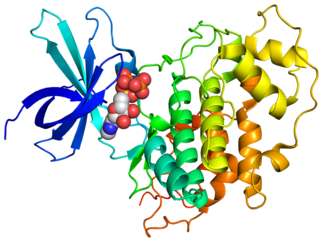
Glycogen synthase kinase 3 (GSK-3) is a serine/threonine protein kinase that mediates the addition of phosphate molecules onto serine and threonine amino acid residues. First discovered in 1980 as a regulatory kinase for its namesake, glycogen synthase (GS), GSK-3 has since been identified as a protein kinase for over 100 different proteins in a variety of different pathways. In mammals, including humans, GSK-3 exists in two isozymes encoded by two homologous genes GSK-3α (GSK3A) and GSK-3β (GSK3B). GSK-3 has been the subject of much research since it has been implicated in a number of diseases, including type 2 diabetes, Alzheimer's disease, inflammation, cancer, addiction and bipolar disorder.
In organic chemistry, a dicarboxylic acid is an organic compound containing two carboxyl groups. The general molecular formula for dicarboxylic acids can be written as HO2C−R−CO2H, where R can be aliphatic or aromatic. In general, dicarboxylic acids show similar chemical behavior and reactivity to monocarboxylic acids.

Neuroprotection refers to the relative preservation of neuronal structure and/or function. In the case of an ongoing insult the relative preservation of neuronal integrity implies a reduction in the rate of neuronal loss over time, which can be expressed as a differential equation. It is a widely explored treatment option for many central nervous system (CNS) disorders including neurodegenerative diseases, stroke, traumatic brain injury, spinal cord injury, and acute management of neurotoxin consumption. Neuroprotection aims to prevent or slow disease progression and secondary injuries by halting or at least slowing the loss of neurons. Despite differences in symptoms or injuries associated with CNS disorders, many of the mechanisms behind neurodegeneration are the same. Common mechanisms of neuronal injury include decreased delivery of oxygen and glucose to the brain, energy failure, increased levels in oxidative stress, mitochondrial dysfunction, excitotoxicity, inflammatory changes, iron accumulation, and protein aggregation. Of these mechanisms, neuroprotective treatments often target oxidative stress and excitotoxicity—both of which are highly associated with CNS disorders. Not only can oxidative stress and excitotoxicity trigger neuron cell death but when combined they have synergistic effects that cause even more degradation than on their own. Thus limiting excitotoxicity and oxidative stress is a very important aspect of neuroprotection. Common neuroprotective treatments are glutamate antagonists and antioxidants, which aim to limit excitotoxicity and oxidative stress respectively.

Zeaxanthin is one of the most common carotenoids in nature, and is used in the xanthophyll cycle. Synthesized in plants and some micro-organisms, it is the pigment that gives paprika, corn, saffron, goji (wolfberries), and many other plants and microbes their characteristic color.

Crocus sativus, commonly known as saffron crocus or autumn crocus, is a species of flowering plant in the iris family Iridaceae. A cormous autumn-flowering cultivated perennial, unknown in the wild, it is best known for the culinary use of its floral stigmas as the spice saffron. Human cultivation of saffron crocus and the trade and use of saffron have endured for more than 3,500 years and span different cultures, continents, and civilizations.

Safranal is an organic compound isolated from saffron, the spice consisting of the stigmas of crocus flowers. It is the constituent primarily responsible for the aroma of saffron.

Picrocrocin is a monoterpene glycoside precursor of safranal. It is found in the spice saffron, which comes from the crocus flower. Picrocrocin has a bitter taste, and is the chemical most responsible for the taste of saffron.

Crocus cartwrightianus is a species of flowering plant in the family Iridaceae, native to mainland Greece and Crete. It is a cormous perennial growing to 5 cm (2 in). The flowers, in shades of lilac or white with purple veins and prominent red stigmas, appear with the leaves in autumn and winter.

Crocetin is a natural apocarotenoid dicarboxylic acid that is found in the crocus flower together with its glycoside, crocin, and Gardenia jasminoides fruits. It is also known as crocetic acid. It forms brick red crystals with a melting point of 285°C.

Genistein (C15H10O5) is a naturally occurring compound that structurally belongs to a class of compounds known as isoflavones. It is described as an angiogenesis inhibitor and a phytoestrogen.
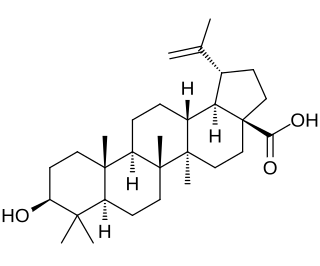
Betulinic acid is a naturally occurring pentacyclic triterpenoid which has antiretroviral, antimalarial, and anti-inflammatory properties, as well as a more recently discovered potential as an anticancer agent, by inhibition of topoisomerase. It is found in the bark of several species of plants, principally the white birch from which it gets its name, but also the ber tree, selfheal, the tropical carnivorous plants Triphyophyllum peltatum and Ancistrocladus heyneanus, Diospyros leucomelas, a member of the persimmon family, Tetracera boiviniana, the jambul, flowering quince, rosemary, and Pulsatilla chinensis.

Nuclear factor erythroid 2-related factor 2 (NRF2), also known as nuclear factor erythroid-derived 2-like 2, is a transcription factor that in humans is encoded by the NFE2L2 gene. NRF2 is a basic leucine zipper (bZIP) protein that may regulate the expression of antioxidant proteins that protect against oxidative damage triggered by injury and inflammation, according to preliminary research. In vitro, NRF2 binds to antioxidant response elements (AREs) in the promoter regions of genes encoding cytoprotective proteins. NRF2 induces the expression of heme oxygenase 1 in vitro leading to an increase in phase II enzymes. NRF2 also inhibits the NLRP3 inflammasome.
Divicine (2,6-diamino-4,5-dihydroxypyrimidine) is an oxidant and a base with alkaloidal properties found in fava beans and Lathyrus sativus. It is an aglycone of vicine. A common derivative is the diacetate form (2,6-diamino-1,6-dihydro-4,5-pyrimidinedione).

Valorphin, also known as VV-hemorphin-5, is a naturally occurring, endogenous opioid heptapeptide of the hemorphin family with the amino acid sequence H-Val-Val-Tyr-Pro-Trp-Thr-Gln-OH (VVYPWTQ). It is produced in the body via proteolyic cleavage of residues 33-39 of the β-chain of hemoglobin. Valorphin binds preferentially to the μ-opioid receptor and produces effects such as analgesia and self-administration in animals. It also possesses cytotoxic and antiproliferative properties against tumor cells, the mediation of which, because they are reversed by naloxone, appears to be dependent on the opioid receptors.
An oxygen diffusion-enhancing compound is any substance that increases the availability of oxygen in body tissues by influencing the molecular structure of water in blood plasma and thereby promoting the movement (diffusion) of oxygen through plasma. Oxygen diffusion-enhancing compounds have shown promise in the treatment of conditions associated with hypoxia and ischemia. Such conditions include hemorrhagic shock, myocardial infarction, and stroke.
Crocetin glucosyltransferase is an enzyme with systematic name UDP-glucose:crocetin 8-O-D-glucosyltransferase. This enzyme catalyses the following chemical reaction
Saffron is one of the world's most expensive spices by weight due to its difficulty to harvest. Saffron consists of stigmas plucked from the vegetatively propagated and sterile Crocus sativus, known popularly as the saffron crocus. The resulting dried "threads" are distinguished by their bitter taste, hay-like fragrance, and slight metallic notes. The saffron crocus is unknown in the wild; its most likely precursor, Crocus cartwrightianus, originated in Crete or Central Asia; The saffron crocus is native to Southwest Asia, and is believed to have been first cultivated in Iran. Greece, Turkey, and Kashmir (India) have also been suggested as possible sites of origin.
"Saffron, for example, was once less regarded than it is today because the crocus from which it is extracted was not particularly mysterious. It flourished in European locations extending from Asia Minor, where it originated, to Saffron Walden in England, where it was naturalised. Only subsequently, when its labour-intensive cultivation became largely centred in Kashmir (India), did it seem sufficiently exotic to qualify as one of the most precious of spices."

DDB1- and CUL4-associated factor 11 also known as WD Repeat Domain 23 (WDR23) is a protein that in humans is encoded by the DCAF11 gene.















