In chemistry, an acyl group is a moiety derived by the removal of one or more hydroxyl groups from an oxoacid, including inorganic acids. It contains a double-bonded oxygen atom and an organyl group or hydrogen in the case of formyl group. In organic chemistry, the acyl group is usually derived from a carboxylic acid, in which case it has the formula R−C(=O)−, where R represents an organyl group or hydrogen. Although the term is almost always applied to organic compounds, acyl groups can in principle be derived from other types of acids such as sulfonic acids and phosphonic acids. In the most common arrangement, acyl groups are attached to a larger molecular fragment, in which case the carbon and oxygen atoms are linked by a double bond.

The cyanate ion is an anion with the chemical formula OCN−. It is a resonance of three forms: [O−−C≡N] (61%) ↔ [O=C=N−] (30%) ↔ [O+≡C−N2−] (4%).

18-Crown-6 is an organic compound with the formula [C2H4O]6 and the IUPAC name of 1,4,7,10,13,16-hexaoxacyclooctadecane. It is a white, hygroscopic crystalline solid with a low melting point. Like other crown ethers, 18-crown-6 functions as a ligand for some metal cations with a particular affinity for potassium cations (binding constant in methanol: 106 M−1). The point group of 18-crown-6 is S6. The dipole moment of 18-crown-6 varies in different solvent and under different temperature. Under 25 °C, the dipole moment of 18-crown-6 is 2.76 ± 0.06 D in cyclohexane and 2.73 ± 0.02 in benzene. The synthesis of the crown ethers led to the awarding of the Nobel Prize in Chemistry to Charles J. Pedersen.

A carbenium ion is a positive ion with the structure RR′R″C+, that is, a chemical species with carbon atom having three covalent bonds, and it bears a +1 formal charge. But IUPAC confuses coordination number with valence, incorrectly considering carbon in carbenium as trivalent.
Pyrylium is a cation with formula C5H5O+, consisting of a six-membered ring of five carbon atoms, each with one hydrogen atom, and one positively charged oxygen atom. The bonds in the ring are conjugated as in benzene, giving it an aromatic character. In particular, because of the positive charge, the oxygen atom is trivalent. Pyrilium is a mono-cyclic and heterocyclic compound, one of the oxonium ions.
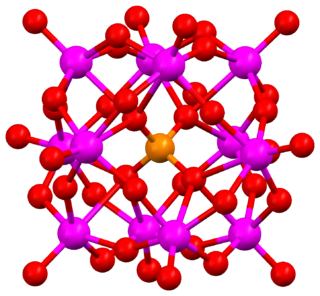
The Keggin structure is the best known structural form for heteropoly acids. It is the structural form of α-Keggin anions, which have a general formula of [XM12O40]n−, where X is the heteroatom, M is the addendum atom, and O represents oxygen. The structure self-assembles in acidic aqueous solution and is a commonly used type of polyoxometalate catalysts.

Potassium ferrioxalate, also called potassium trisoxalatoferrate or potassium tris(oxalato)ferrate(III) is a chemical compound with the formula K3[Fe(C2O4)3]. It often occurs as the trihydrate K3[Fe(C2O4)3]·3H2O. Both are crystalline compounds, lime green in colour.
A carbon–oxygen bond is a polar covalent bond between atoms of carbon and oxygen. Carbon–oxygen bonds are found in many inorganic compounds such as carbon oxides and oxohalides, carbonates and metal carbonyls, and in organic compounds such as alcohols, ethers, carbonyl compounds and oxalates. Oxygen has 6 valence electrons of its own and tends to fill its outer shell with 8 electrons by sharing electrons with other atoms to form covalent bonds, accepting electrons to form an anion, or a combination of the two. In neutral compounds, an oxygen atom can form up to two single bonds or one double bond with carbon, while a carbon atom can form up to four single bonds or two double bonds with oxygen.

Tetrahydroxy-1,4-benzoquinone, also called tetrahydroxy-p-benzoquinone, tetrahydroxybenzoquinone, or tetrahydroxyquinone, is an organic compound with formula C6O2(OH)4. Its molecular structure consists of a cyclohexadiene ring with four hydroxyl groups and two ketone groups in opposite (para) positions.
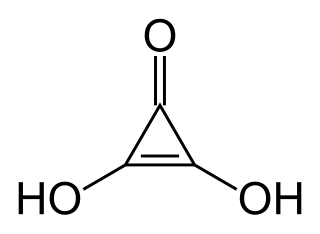
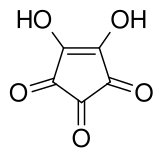
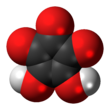
Deltic acid is a chemical substance with the chemical formula C3O(OH)2. It can be viewed as a ketone and double enol of cyclopropene. At room temperature, it is a stable white solid, soluble in diethyl ether, that decomposes between 140 °C and 180 °C, and reacts slowly with water.
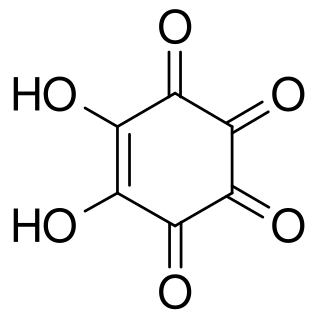
Rhodizonic acid is a chemical compound with formula H2C6O6 or (CO)4(COH)2. It can be seen as a twofold enol and fourfold ketone of cyclohexene, more precisely 5,6-dihydroxycyclohex-5-ene-1,2,3,4-tetrone.

A disulfite, commonly known as metabisulfite or pyrosulfite, is a chemical compound containing the ion S
2O2−
5. It is a colorless dianion that is primarily marketed in the form of sodium metabisulfite or potassium metabisulfite. When dissolved in water, these salts release the hydrogensulfite HSO−
3 anion. These salts act equivalently to sodium hydrogensulfite or potassium hydrogensulfite.

Croconate violet or 1,3-bis(dicyanomethylene)croconate is a divalent anion with chemical formula C
11N
4O2−
3 or ((N≡C−)2C=)2(C5O3)2−. It is one of the pseudo-oxocarbon anions, as it can be described as a derivative of the croconate oxocarbon anion C
5O2−
5 through the replacement of two oxygen atoms by dicyanomethylene groups =C(−C≡N)2. Its systematic name is 3,5-bis(dicyanomethylene)-1,2,4-trionate. The term croconate violet as a dye name specifically refers to the dipotassium salt K
2C
11N
4O
3.

1,2-Bis(dicyanomethylene)squarate is a divalent anion with chemical formula C
10N
4O2−
2 or ((N≡C−)2C=)2(C4O2)2−. It is one of the pseudo-oxocarbon anions, as it can be described as a derivative of the squarate oxocarbon anion C
4O2−
4 through the replacement of two adjacent oxygen atoms by dicyanomethylene groups =C(−C≡N)2.

1,3-Bis(dicyanomethylene)squarate is a divalent anion with chemical formula C
10N
4O2−
2 or ((N≡C−)2C=)2(C4O2)2−. It is one of the pseudo-oxocarbon anions, as it can be described as a derivative of the squarate oxocarbon anion C
4O2−
4 through the replacement of two opposite oxygen atoms by dicyanomethylene groups =C(−C≡N)2.
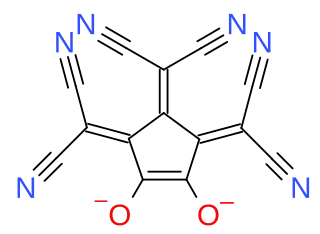
Croconate blue or 1,2,3-tris(dicyanomethylene)croconate is a divalent anion with chemical formula C
14N
6O2−
2 or ((N≡C−)2C=)3(C5O2)2−. It is one of the pseudo-oxocarbon anions, as it can be described as a derivative of the croconate oxocarbon anion C
5O2−
5 through the replacement of three oxygen atoms by dicyanomethylene groups =C(−C≡N)2. The term Croconate Blue as a dye name specifically refers to the dipotassium salt K
2C
14N
6O
2.

2-(Dicyanomethylene)croconate is a divalent anion with chemical formula C
8N
2O2−
4 or ((N≡C−)2C=)(C5O4)2−. It is one of the pseudo-oxocarbon anions, as it can be described as a derivative of the croconate oxocarbon anion C
5O2−
5 through the replacement of one oxygen atom by a dicyanomethylene group =C(−C≡N)2.

In chemistry, the term pseudo-oxocarbon anion is used to refer to a negative ion that is conceptually derived from an oxocarbon anion through replacement of one or more of the basic oxygen atoms by chemically similar elements or functional groups, such as sulfur (S), selenium (Se), or dicyanomethylene (=C(CN)2).

Cerium nitrate refers to a family of nitrates of cerium in the +3 or +4 oxidation state. Often these compounds contain water, hydroxide, or hydronium ions in addition to cerium and nitrate. Double nitrates of cerium also exist.
The nickel organic acid salts are organic acid salts of nickel. In many of these the ionised organic acid acts as a ligand.