
In physics, cryogenics is the production and behaviour of materials at very low temperatures.

Liquid hydrogen (H2(l)) is the liquid state of the element hydrogen. Hydrogen is found naturally in the molecular H2 form.

A vacuum flask is an insulating storage vessel that slows the speed at which its contents change in temperature. It greatly lengthens the time over which its contents remain hotter or cooler than the flask's surroundings by trying to be as adiabatic as possible. Invented by Sir James Dewar in 1892, the vacuum flask consists of two flasks, placed one within the other and joined at the neck. The gap between the two flasks is partially evacuated of air, creating a near-vacuum which significantly reduces heat transfer by conduction or convection. When used to hold cold liquids, this also virtually eliminates condensation on the outside of the flask.
A cryopump or a "cryogenic pump" is a vacuum pump that traps gases and vapours by condensing them on a cold surface, but are only effective on some gases. The effectiveness depends on the freezing and boiling points of the gas relative to the cryopump's temperature. They are sometimes used to block particular contaminants, for example in front of a diffusion pump to trap backstreaming oil, or in front of a McLeod gauge to keep out water. In this function, they are called a cryotrap, waterpump or cold trap, even though the physical mechanism is the same as for a cryopump.

Liquid nitrogen—LN2—is nitrogen in a liquid state at low temperature. Liquid nitrogen has a boiling point of about −196 °C (−321 °F; 77 K). It is produced industrially by fractional distillation of liquid air. It is a colorless, mobile liquid whose viscosity is about one tenth that of acetone (i.e. roughly one thirtieth that of room temperature water). Liquid nitrogen is widely used as a coolant.
Cryogenic fuels are fuels that require storage at extremely low temperatures in order to maintain them in a liquid state. These fuels are used in machinery that operates in space where ordinary fuel cannot be used, due to the very low temperatures often encountered in space, and the absence of an environment that supports combustion. Cryogenic fuels most often constitute liquefied gases such as liquid hydrogen.

Bottled gas is a term used for substances which are gaseous at standard temperature and pressure (STP) and have been compressed and stored in carbon steel, stainless steel, aluminum, or composite containers known as gas cylinders.
Liquid air is air that has been cooled to very low temperatures, so that it has condensed into a pale blue mobile liquid. It is stored in specialized containers, such as vacuum flasks, to insulate it from room temperature. Liquid air can absorb heat rapidly and revert to its gaseous state. It is often used for condensing other substances into liquid and/or solidifying them, and as an industrial source of nitrogen, oxygen, argon, and other inert gases through a process called air separation.
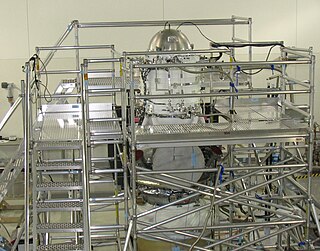
A cryostat is a device used to maintain low cryogenic temperatures of samples or devices mounted within the cryostat. Low temperatures may be maintained within a cryostat by using various refrigeration methods, most commonly using cryogenic fluid bath such as liquid helium. Hence it is usually assembled into a vessel, similar in construction to a vacuum flask or Dewar. Cryostats have numerous applications within science, engineering, and medicine.

Laboratory flasks are vessels or containers that fall into the category of laboratory equipment known as glassware. In laboratory and other scientific settings, they are usually referred to simply as flasks. Flasks come in a number of shapes and a wide range of sizes, but a common distinguishing aspect in their shapes is a wider vessel "body" and one narrower tubular sections at the top called necks which have an opening at the top. Laboratory flask sizes are specified by the volume they can hold, typically in metric units such as milliliters or liters. Laboratory flasks have traditionally been made of glass, but can also be made of plastic.
The sorption pump is a vacuum pump that creates a vacuum by adsorbing molecules on a very porous material like molecular sieve which is cooled by a cryogen, typically liquid nitrogen. The ultimate pressure is about 10−2 mbar. With special techniques this can be lowered till 10−7 mbar. The main advantages are the absence of oil or other contaminants, low cost and vibration free operation because there are no moving parts. The main disadvantages are that it cannot operate continuously and cannot effectively pump hydrogen, helium and neon, all gases with lower condensation temperature than liquid nitrogen. The main application is as a roughing pump for a sputter-ion pump in ultra-high vacuum experiments, for example in surface physics.

An LNG carrier is a tank ship designed for transporting liquefied natural gas (LNG).
A refrigerated transport Dewar is a refrigerated transport vessel with an insulated Dewar flask (vacuum) design to carry cryogenic liquid. To prevent pressure build-up they are equipped with safety relief valves and/or rupture discs. The liquid can be withdrawn as a gas by passing liquid through an internal vaporizer or as a liquid under its own vapour pressure.
A liquid nitrogen vehicle is powered by liquid nitrogen, which is stored in a tank. Traditional nitrogen engine designs work by heating the liquid nitrogen in a heat exchanger, extracting heat from the ambient air and using the resulting pressurized gas to operate a piston or rotary motor. Vehicles propelled by liquid nitrogen have been demonstrated, but are not used commercially. One such vehicle, Liquid Air, was demonstrated in 1902.
An air separation plant separates atmospheric air into its primary components, typically nitrogen and oxygen, and sometimes also argon and other rare inert gases.

A cryogenic gas plant is an industrial facility that creates molecular oxygen, molecular nitrogen, argon, krypton, helium, and xenon at relatively high purity. As air is made up of nitrogen, the most common gas in the atmosphere, at 78%, with oxygen at 19%, and argon at 1%, with trace gasses making up the rest, cryogenic gas plants separate air inside a distillation column at cryogenic temperatures to produce high purity gasses such as argon, nitrogen, oxygen, and many more with 1 ppm or less impurities. The process is based on the general theory of the Hampson-Linde cycle of air separation, which was invented by Carl von Linde in 1895.

A gas carrier, gas tanker, LPG carrier, or LPG tanker is a ship designed to transport LPG, LNG, CNG, or liquefied chemical gases in bulk.
A Horton sphere, also referred to as a spherical tank or simply sphere, is a spherical pressure vessel, which is used for industrial-scale storage of liquefied gases. Example of materials that can be stored in Horton spheres are liquefied petroleum gas (LPG), liquefied natural gas (LNG), and anhydrous ammonia.

A liquefied natural gas terminal is a facility for managing the import and/or export of liquefied natural gas (LNG). It comprises equipment for loading and unloading of LNG cargo to/from ocean-going tankers, for transfer across the site, liquefaction, re-gasification, processing, storage, pumping, compression, and metering of LNG. LNG as a liquid is the most efficient way to transport natural gas over long distances, usually by sea.
Many laboratories contain significant risks, and the prevention of laboratory accidents requires great care and constant vigilance. Examples of risk factors include high voltages, high and low pressures and temperatures, corrosive and toxic chemicals and chemical vapours, radiation, fire, explosions, and biohazards including infective organisms and their toxins.










