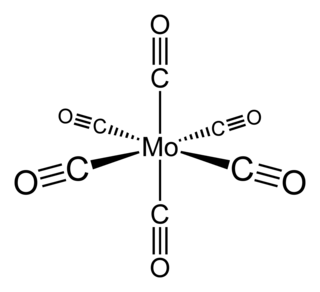
Molybdenum hexacarbonyl (also called molybdenum carbonyl) is the chemical compound with the formula Mo(CO)6. This colorless solid, like its chromium and tungsten analogues, is noteworthy as a volatile, air-stable derivative of a metal in its zero oxidation state.

Iron pentacarbonyl, also known as iron carbonyl, is the compound with formula Fe(CO)5. Under standard conditions Fe(CO)5 is a free-flowing, straw-colored liquid with a pungent odour. Older samples appear darker. This compound is a common precursor to diverse iron compounds, including many that are useful in small scale organic synthesis.
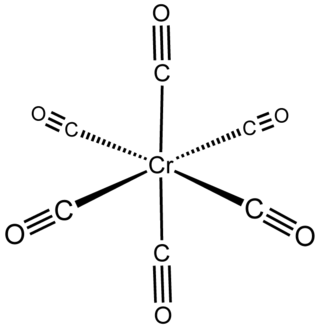
Chromium carbonyl, also known as chromium hexacarbonyl, is the chemical compound with the formula Cr(CO)6. At room temperature the solid is stable to air, although it does have a high vapor pressure and sublimes readily. Cr(CO)6 is zerovalent, meaning that Cr has an oxidation state of zero, and it is a homoleptic complex, which means that all the ligands are identical. The complex is octahedral with Cr–C and C–O distances of 1.91 and 1.14 Å, respectively.
Cycloheptatriene (CHT) is an organic compound with the formula C7H8. It is a closed ring of seven carbon atoms joined by three double bonds (as the name implies) and four single bonds. This colourless liquid has been of recurring theoretical interest in organic chemistry. It is a ligand in organometallic chemistry and a building block in organic synthesis. Cycloheptatriene is not aromatic, as reflected by the nonplanarity of the methylene bridge (-CH2-) with respect to the other atoms; however the related tropylium cation is.
In organic chemistry, the tropylium ion or cycloheptatrienyl cation is an aromatic species with a formula of [C7H7]+. Its name derives from the molecule tropine from which cycloheptatriene (tropylidene) was first synthesized in 1881. Salts of the tropylium cation can be stable, even with nucleophiles of moderate strength e.g., tropylium tetrafluoroborate and tropylium bromide (see below). Its bromide and chloride salts can be made from cycloheptatriene and bromine or phosphorus pentachloride, respectively.
Homoaromaticity, in organic chemistry, refers to a special case of aromaticity in which conjugation is interrupted by a single sp3 hybridized carbon atom. Although this sp3 center disrupts the continuous overlap of p-orbitals, traditionally thought to be a requirement for aromaticity, considerable thermodynamic stability and many of the spectroscopic, magnetic, and chemical properties associated with aromatic compounds are still observed for such compounds. This formal discontinuity is apparently bridged by p-orbital overlap, maintaining a contiguous cycle of π electrons that is responsible for this preserved chemical stability.

Tungsten hexacarbonyl (also called tungsten carbonyl) is the chemical compound with the formula W(CO)6. This complex gave rise to the first example of a dihydrogen complex.

Metal nitrosyl complexes are complexes that contain nitric oxide, NO, bonded to a transition metal. Many kinds of nitrosyl complexes are known, which vary both in structure and coligand.

Triruthenium dodecacarbonyl is the chemical compound with the formula Ru3(CO)12. Classified as metal carbonyl cluster, it is a dark orange-colored solid that is soluble in nonpolar organic solvents. The compound serves as a precursor to other organoruthenium compounds.
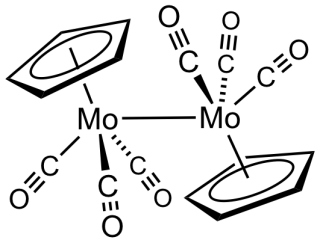
Cyclopentadienylmolybdenum tricarbonyl dimer is the chemical compound with the formula Cp2Mo2(CO)6, where Cp is C5H5. A dark red solid, it has been the subject of much research although it has no practical uses.
Organoiron chemistry is the chemistry of iron compounds containing a carbon-to-iron chemical bond. Organoiron compounds are relevant in organic synthesis as reagents such as iron pentacarbonyl, diiron nonacarbonyl and disodium tetracarbonylferrate. While iron adopts oxidation states from Fe(−II) through to Fe(VII), Fe(IV) is the highest established oxidation state for organoiron species. Although iron is generally less active in many catalytic applications, it is less expensive and "greener" than other metals. Organoiron compounds feature a wide range of ligands that support the Fe-C bond; as with other organometals, these supporting ligands prominently include phosphines, carbon monoxide, and cyclopentadienyl, but hard ligands such as amines are employed as well.

Organomolybdenum chemistry is the chemistry of chemical compounds with Mo-C bonds. The heavier group 6 elements molybdenum and tungsten form organometallic compounds similar to those in organochromium chemistry but higher oxidation states tend to be more common.

Molybdenum(II) acetate is a coordination compound with the formula Mo2(O2CCH3)4. It is a yellow, diamagnetic, air-stable solid that is slightly soluble in organic solvents. Molybdenum(II) acetate is an iconic example of a compound with a metal-metal quadruple bond.

(Mesitylene)molybdenum tricarbonyl is an organomolybdenum compound derived from the aromatic compound mesitylene (1,3,5-trimethylbenzene) and molybdenum carbonyl. It exists as pale yellow crystals, which are soluble in organic solvents but decompose when in solution. It has been examined as a catalyst and reagent.

Half sandwich compounds, also known as piano stool complexes, are organometallic complexes that feature a cyclic polyhapto ligand bound to an MLn center, where L is a unidentate ligand. Thousands of such complexes are known. Well-known examples include cyclobutadieneiron tricarbonyl and (C5H5)TiCl3. Commercially useful examples include (C5H5)Co(CO)2, which is used in the synthesis of substituted pyridines, and methylcyclopentadienyl manganese tricarbonyl, an antiknock agent in petrol.
In organometallic chemistry, a transition metal alkene complex is a coordination compound containing one or more alkene ligands. Such compounds are intermediates in many catalytic reactions that convert alkenes to other organic products.

Transition metal nitrile complexes are coordination compounds containing nitrile ligands. Because nitriles are weakly basic, the nitrile ligands in these complexes are often labile.

Cyclopentadienyltungsten tricarbonyl dimer is the organotungsten compound with the formula Cp2W2(CO)6, where Cp is C5H5. A dark red crystalline solid, it is the subject of research, although it has no or few practical uses.

Cyclopentadienylchromium tricarbonyl dimer is the organochromium compound with the formula Cp2Cr2(CO)6, where Cp is C5H5. A dark green crystalline solid. It is the subject of research it exists in measureable equilibrium quantities with the monometallic radical CpCr(CO)3.
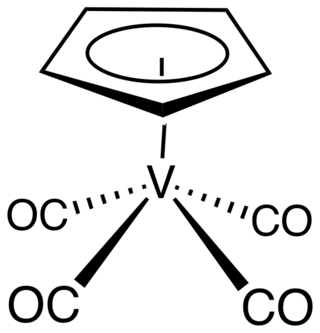
Cyclopentadienylvanadium tetracarbonyl is the organovanadium compound with the formula (C5H5)V(CO)4. An orange, diamagnetic solid, it is the principal cyclopentadienyl carbonyl of vanadium. It can be prepared by heating a solution of vanadocene under high pressure of carbon monoxide. As confirmed by X-ray crystallography, the coordination sphere of vanadium consists of η5-cyclopentadienyl and four carbonyl ligands. The molecule is a four-legged piano stool complex. The compound is soluble in common organic solvents. The compound has no commercial applications.

















