Related Research Articles

Dichlorodiphenyltrichloroethane, commonly known as DDT, is a colorless, tasteless, and almost odorless crystalline chemical compound, an organochloride. Originally developed as an insecticide, it became infamous for its environmental impacts. DDT was first synthesized in 1874 by the Austrian chemist Othmar Zeidler. DDT's insecticidal action was discovered by the Swiss chemist Paul Hermann Müller in 1939. DDT was used in the second half of World War II to limit the spread of the insect-borne diseases malaria and typhus among civilians and troops. Müller was awarded the Nobel Prize in Physiology or Medicine in 1948 "for his discovery of the high efficiency of DDT as a contact poison against several arthropods".

Pesticides are substances that are meant to control pests. The term pesticide includes all of the following: herbicide, insecticides nematicide, molluscicide, piscicide, avicide, rodenticide, bactericide, insect repellent, animal repellent, antimicrobial, fungicide, and lampricide. The most common of these are herbicides which account for approximately 80% of all pesticide use. Most pesticides are intended to serve as plant protection products, which in general, protect plants from weeds, fungi, or insects. As an example, the fungus Alternaria solani is used to combat the aquatic weed Salvinia.
Chlordane, or chlordan, is an organochlorine compound that was used as a pesticide. It is a white solid. In the United States, chlordane was used for termite-treatment of approximately 30 million homes until it was banned in 1988. Chlordane was banned 10 years earlier for food crops like corn and citrus, and on lawns and domestic gardens.
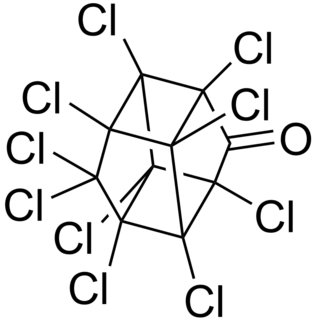
Chlordecone, better known in the United States under the brand name Kepone, is an organochlorine compound and a colourless solid. It is an obsolete insecticide, related to Mirex and DDT. Its use was so disastrous that it is now prohibited in the western world, but only after many thousands of tonnes had been produced and used. Chlordecone is a known persistent organic pollutant (POP) that was banned globally by the Stockholm Convention on Persistent Organic Pollutants in 2009.

Lindane, also known as gamma-hexachlorocyclohexane (γ-HCH), gammaxene, Gammallin and sometimes misnamed benzene hexachloride (BHC), is an organochlorine chemical and an isomer of hexachlorocyclohexane that has been used both as an agricultural insecticide and as a pharmaceutical treatment for lice and scabies.

Persistent organic pollutants (POPs), sometimes known as "forever chemicals", are organic compounds that are resistant to environmental degradation through chemical, biological, and photolytic processes. They are toxic chemicals that adversely affect human health and the environment around the world. Because they can be transported by wind and water, most POPs generated in one country can and do affect people and wildlife far from where they are used and released. The effect of POPs on human and environmental health was discussed, with intention to eliminate or severely restrict their production, by the international community at the Stockholm Convention on Persistent Organic Pollutants in 2001. The United States has taken strong domestic action to reduce emissions of POPs. For example, none of the original POPs pesticides listed in the Stockholm Convention is registered for sale and distribution in the United States today and in 1978, Congress prohibited the manufacture of polychlorinated biphenyl (PCB) and severely restricted the use of remaining PCB stocks. In addition, since 1987, the Environmental Protection Agency and the states have effectively reduced environmental releases of dioxins and furans to land, air, and water from U.S. sources.

Dieldrin is an organochloride originally produced in 1948 by J. Hyman & Co, Denver, as an insecticide. Dieldrin is closely related to aldrin, which reacts further to form dieldrin. Aldrin is not toxic to insects; it is oxidized in the insect to form dieldrin which is the active compound. Both dieldrin and aldrin are named after the Diels-Alder reaction which is used to form aldrin from a mixture of norbornadiene and hexachlorocyclopentadiene.

Aldrin is an organochlorine insecticide that was widely used until the 1990s, when it was banned in most countries. Aldrin is a member of the so-called "classic organochlorines" (COC) group of pesticides. COCs enjoyed a very sharp rise in popularity during and after The Second World War. Other noteworthy examples of COCs include DDT. After research showed that organochlorines can be highly toxic to the ecosystem through bioaccumulation, most were banned from use. It is a colourless solid. Before the ban, it was heavily used as a pesticide to treat seed and soil. Aldrin and related "cyclodiene" pesticides became notorious as persistent organic pollutants.

Endosulfan is an off-patent organochlorine insecticide and acaricide that is being phased out globally. It became a highly controversial agrichemical due to its acute toxicity, potential for bioaccumulation, and role as an endocrine disruptor. Because of its threats to human health and the environment, a global ban on the manufacture and use of endosulfan was negotiated under the Stockholm Convention in April 2011. The ban has taken effect in mid-2012, with certain uses exempted for five additional years. More than 80 countries, including the European Union, Australia, New Zealand, several West African nations, the United States, Brazil, and Canada had already banned it or announced phase-outs by the time the Stockholm Convention ban was agreed upon. It is still used extensively in India, China despite laws banning it, and few other countries. It is produced by Makhteshim Agan and several manufacturers in India and China. Although, the Supreme Court had, by an order dated 13.05.2011, put a ban on the production and sale of endosulfan in India till further orders.
Pesticide residue refers to the pesticides that may remain on or in food after they are applied to food crops. The maximum allowable levels of these residues in foods are often stipulated by regulatory bodies in many countries. Regulations such as pre-harvest intervals also often prevent harvest of crop or livestock products if recently treated in order to allow residue concentrations to decrease over time to safe levels before harvest. Exposure of the general population to these residues most commonly occurs through consumption of treated food sources, or being in close contact to areas treated with pesticides such as farms or lawns.

Endrin is an organochloride with the chemical formula C12H8Cl6O that was first produced in 1950 by Shell and Velsicol Chemical Corporation. It was primarily used as an insecticide, as well as a rodenticide and piscicide. It is a colourless, odorless solid, although commercial samples are often off-white. Endrin was manufactured as an emulsifiable solution known commercially as Endrex. The compound became infamous as a persistent organic pollutant and for this reason it is banned in many countries.

β-Hexachlorocyclohexane (β-HCH) is an organochloride which is one of the isomers of hexachlorocyclohexane (HCH). It is a byproduct of the production of the insecticide lindane (γ-HCH). It is typically constitutes 5–14% of technical-grade lindane, though it has not been produced or used in the United States since 1985. As of 2009, the Stockholm Convention on Persistent Organic Pollutants classified α-hexachlorocyclohexane and β-HCH as persistent organic pollutants (POPs), due to the chemical's ability to persist in the environment, bioaccumulative, biomagnifying, and long-range transport capacity.

Mirex was an organochloride that was commercialized as an insecticide and later banned because of its impact on the environment. This white crystalline odorless solid is a derivative of cyclopentadiene. It was popularized to control fire ants but by virtue of its chemical robustness and lipophilicity it was recognized as a bioaccumulative pollutant. The spread of the red imported fire ant was encouraged by the use of Mirex, which also kills native ants that are highly competitive with the fire ants. The United States Environmental Protection Agency prohibited its use in 1976. It is prohibited by the Stockholm Convention on Persistent Organic Pollutants.

Soil contamination, soil pollution, or land pollution as a part of land degradation is caused by the presence of xenobiotic (human-made) chemicals or other alteration in the natural soil environment. It is typically caused by industrial activity, agricultural chemicals or improper disposal of waste. The most common chemicals involved are petroleum hydrocarbons, polynuclear aromatic hydrocarbons, solvents, pesticides, lead, and other heavy metals. Contamination is correlated with the degree of industrialization and intensity of chemical substance. The concern over soil contamination stems primarily from health risks, from direct contact with the contaminated soil, vapour from the contaminants, or from secondary contamination of water supplies within and underlying the soil. Mapping of contaminated soil sites and the resulting cleanups are time-consuming and expensive tasks, and require expertise in geology, hydrology, chemistry, computer modeling, and GIS in Environmental Contamination, as well as an appreciation of the history of industrial chemistry.

Environmental toxicology is a multidisciplinary field of science concerned with the study of the harmful effects of various chemical, biological and physical agents on living organisms. Ecotoxicology is a subdiscipline of environmental toxicology concerned with studying the harmful effects of toxicants at the population and ecosystem levels.

Māpua is a small town in the South Island of New Zealand. It is to the west of Nelson on State Highway 60 and on the coastline of Tasman Bay.

The environmental effects of pesticides describe the broad series of consequences of using pesticides. The unintended consequences of pesticides is one of the main drivers of the negative impact of modern industrial agriculture on the environment. Pesticides, because they are toxic chemicals meant to kill pest species, can effect non-target species, such as plants, animals and humans. Over 98% of sprayed insecticides and 95% of herbicides reach a destination other than their target species, because they are sprayed or spread across entire agricultural fields. Other agrochemicals, such as fertilizers, can also have negative effects on the environment.

Pentachlorobenzene (PeCB) is a chemical compound with the molecular formula C6HCl5 which is a chlorinated aromatic hydrocarbon. It consists of a benzene ring substituted with five chlorine atoms. PeCB was once used industrially for a variety of uses, but because of environmental concerns there are currently no large scale uses of PeCB. Pentachlorobenzene is a known persistent organic pollutant (POP) and banned globally by the Stockholm Convention on Persistent Organic Pollutants in 2009.
Persistent, bioaccumulative and toxic substances (PBTs) are a class of compounds that have high resistance to degradation from abiotic and biotic factors, high mobility in the environment and high toxicity. Because of these factors PBTs have been observed to have a high order of bioaccumulation and biomagnification, very long retention times in various media, and widespread distribution across the globe. Majority of PBTs in the environment are either created through industry or are unintentional byproducts.
Pesticide standard values are applied worldwide to control pesticide pollution, since pesticides are largely applied in numerous agricultural, commercial, residential, and industrial applications. Usually, pesticide standard value is regulated in residential surface soil, drinking water, foods, and other ecological sections.
References
- ↑ Salmon, J. T. (1959). "Report of Conservation Committee to The Royal Society of New Zealand on The Use and Effects of Modern Insecticides". Transactions and Proceedings of the Royal Society of New Zealand. Royal Society of New Zealand. 86. Retrieved 2008-09-16.
- 1 2 Taylor, Rowan; New Zealand (1997). The State of New Zealand's Environment 1997. Wellington, N.Z: Ministry for the Environment. ISBN 0-478-09000-5.
- ↑ Smith, C M; R J Wilcock (September 1993). Towards Sustainable Agriculture: Freshwater Quality in New Zealand and the Influence of Agriculture (PDF). MAP Policy Technical Paper 93/10. Ministry of Agriculture and Fisheries. ISBN 0-478-07324-0. Archived from the original (PDF) on 2008-10-15. Retrieved 2008-09-16.
- ↑ Parliamentary Commissioner for the Environment (July 2008). Investigation into the remediation of the contaminated site at Mapua. PCE. ISBN 978-1-877274-17-6.[ permanent dead link ]
- ↑ "Stockholm Convention". Ministry for the Environment. Retrieved 2008-09-15.