Consumer education is the preparation of an individual to be capable of making informed decisions when it comes to purchasing products in a consumer culture. It generally covers various consumer goods and services, prices, what the consumer can expect, standard trade practices, etc. While consumer education can help consumers to make more informed decisions, some researchers have found that its effects can drop off over time, suggesting the need for continual education. New dimensions of consumer education are also beginning to emerge as people become more aware of the need for ethical consumerism and sustainable consumer behaviour in our increasingly globalized society.

Diethylstilbestrol (DES), also known as stilbestrol or stilboestrol, is a nonsteroidal estrogen medication, which is presently rarely used. In the past, it was widely used for a variety of indications, including pregnancy support for those with a history of recurrent miscarriage, hormone therapy for menopausal symptoms and estrogen deficiency, treatment of prostate cancer and breast cancer, and other uses. By 2007, it was only used in the treatment of prostate cancer and breast cancer. In 2011, Hoover and colleagues reported on adverse health outcomes linked to DES including infertility, miscarriage, ectopic pregnancy, preeclampsia, preterm birth, stillbirth, infant death, menopause prior to age 45, breast cancer, cervical cancer, and vaginal cancer. While most commonly taken by mouth, DES was available for use by other routes as well, for instance, vaginal, topical, and by injection.
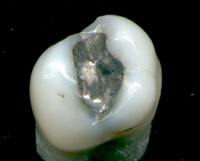
This discussion of the dental amalgam controversy outlines the debate over whether dental amalgam should be used. Supporters claim that it is safe, effective and long-lasting, while critics argue that amalgam is unsafe because it may cause mercury poisoning and other toxicity.
Bioidentical hormone replacement therapy (BHRT), also known as bioidentical hormone therapy(BHT) or natural hormone therapy, is the use of hormones that are identical on a molecular level with endogenous hormones in hormone replacement therapy. It may also be combined with blood and saliva testing of hormone levels, and the use of pharmacy compounding to obtain hormones in an effort to reach a targeted level of hormones in the body. A number of claims by some proponents of BHT have not been confirmed through scientific testing. Specific hormones used in BHT include estrone, estradiol, progesterone, testosterone, dehydroepiandrosterone (DHEA), and estriol.

Sexual and reproductive health (SRH) is a field of research, health care, and social activism that explores the health of an individual's reproductive system and sexual well-being during all stages of their life.
A drug recall removes a prescription or over-the-counter drug from the market. Drug recalls in the United States are made by the FDA or the creators of the drug when certain criteria are met. When a drug recall is made, the drug is removed from the market and potential legal action can be taken depending on the severity of the drug recall.
Peer support occurs when people provide knowledge, experience, emotional, social or practical help to each other. It commonly refers to an initiative consisting of trained supporters, and can take a number of forms such as peer mentoring, reflective listening, or counseling. Peer support is also used to refer to initiatives where colleagues, members of self-help organizations and others meet, in person or online, as equals to give each other connection and support on a reciprocal basis.
The National Women's Health Network (NWHN) is a non-profit women's health advocacy organization located in Washington, D.C. It was founded in 1975 by Barbara Seaman, Alice Wolfson, Belita Cowan, Mary Howell, and Phyllis Chesler. The stated mission of the organization is to give women a greater voice within the healthcare system. The NWHN researches and lobbies federal agencies on such issues as AIDS, reproductive rights, breast cancer, older women's health, and new contraceptive technologies. The Women's Health Voice, the NWHN's health information program, provides independent research on a variety of women's health topics.

Breast Cancer Action (BCAction) is a U.S.-based grassroots education and activist organization driven by and supporting people living with breast cancer. It was founded in 1990 by Elenore Pred, Susan Claymon, and Linda Reyes. Based in San Francisco, BCAction is known for understanding breast cancer not as an individual crisis, but a public health emergency, and for their commitment to social justice. The organization's mission is to achieve health justice for all women at risk of and living with breast cancer. BCAction is known for its Think Before You Pink campaign, launched in 2002, which encourages consumers to ask critical questions before buying pink ribbon products and holds corporations accountable for pinkwashing.
Direct-to-consumer advertising (DTCA) refers to the marketing and advertising of pharmaceutical products directly to consumers as patients, as opposed to specifically targeting health professionals. The term is synonymous primarily with the advertising of prescription medicines via mass media platforms—most commonly on television and in magazines, but also via online platforms.

Dhulikhel Hospital, a Kathmandu University hospital, is an independent, not for profit, non-government hospital in Dhulikhel, Kavrepalanchok, Nepal.

The long-term effects of cannabis have been the subject of ongoing debate. Because cannabis is illegal in most countries, clinical research presents a challenge and there is limited evidence from which to draw conclusions. In 2017, the U.S. National Academies of Sciences, Engineering, and Medicine issued a report summarizing much of the published literature on health effects of cannabis, into categories regarded as conclusive, substantial, moderate, limited and of no or insufficient evidence to support an association with a particular outcome.

Breast cancer awareness is an effort to raise awareness and reduce the stigma of breast cancer through education about screening, symptoms, and treatment. Supporters hope that greater knowledge will lead to earlier detection of breast cancer, which is associated with higher long-term survival rates, and that money raised for breast cancer will produce a reliable, permanent cure.

Fitness to dive, specifically the medical fitness to dive, is the medical and physical suitability of a diver to function safely in the underwater environment using underwater diving equipment and procedures. Depending on the circumstances it may be established by a signed statement by the diver that they do not have any of the listed disqualifying conditions and is able to manage the ordinary physical requirements of diving, to a detailed medical examination by a physician registered as a medical examiner of divers following a procedural checklist, and a legal document of fitness to dive issued by the medical examiner.
Toxic abortion is a medical phenomenon of spontaneous abortion, miscarriage, or stillbirth caused by toxins in the environment of the mother during pregnancy, especially as caused by toxic environmental pollutants, though sometimes reported as caused by naturally occurring plant toxins
Sybil Shainwald is an American attorney specializing in women's health law and an activist for women's health reform. She has represented thousands of women and their children in individual and class action suits against manufacturers of harmful drugs, devices, and procedures. Shainwald is former chair of the National Women's Health Network, co-founder of Health Action International and Trial Lawyers for Public Justice.
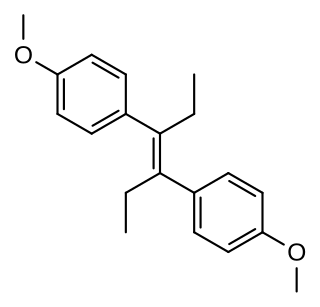
Dimestrol, also known as dianisylhexene, 4,4'-dimethoxy-α,α'-diethylstilbene, diethylstilbestrol dimethyl ether, and dimethoxydiethylstilbestrol, is a synthetic nonsteroidal estrogen of the stilbestrol group which is related to diethylstilbestrol. It has been used clinically as a hormonal therapy in cases of delayed female puberty, hypogonadism, menopausal, and postmenopausal symptoms. It is known to induce the development of female secondary sexual characteristics in the case of female delayed puberty or hypogonadism. The drug has also been used as a growth promoter in livestock.
Diethylstilbestrol (DES), a synthetic nonsteroidal estrogen which was previously used clinically to support pregnancy, has been linked to a variety of long-term adverse effects in women who were treated with it during pregnancy and in their offspring.
Medical reversal refers to when a newer and methodologically superior clinical trial produces results that contradict existing clinical practice and the older trials on which it is based. This leads to an intervention that was widely used falling out of favor, because new evidence either demonstrates that it is ineffective or that its harms exceed its benefits. It is distinct from replacement, which occurs when a newly developed medical treatment supersedes an older, less effective one as the standard of care. Medical reversals are caused when a treatment is widely adopted even when there is not compelling evidence for its safety and effectiveness. For example, an intervention may be adopted because it "makes sense", or because there are observational studies supporting its putative benefits. The negative effects of such reversals include harm to patients who received the intervention when it was considered relatively safe and effective, as well as reducing public trust in medicine.
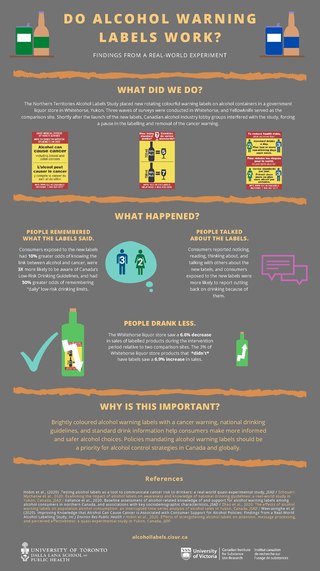
The Northern Territories Alcohol Labels Study was a scientific experiment in Canada on the effects of alcohol warning labels. It was terminated after lobbying from the alcohol industry, and later relaunched with industry-advocated experimental design changes: omitting the "Alcohol can cause cancer" label, not labelling some alcohol products, and shortening the time period. Enough data was gathered to show that all of the labels used in the study were simple, cheap, and effective, and it recommended that they should be required worldwide.










