In chemical analysis, chromatography is a laboratory technique for the separation of a mixture into its components. The mixture is dissolved in a fluid solvent called the mobile phase, which carries it through a system on which a material called the stationary phase is fixed. Because the different constituents of the mixture tend to have different affinities for the stationary phase and are retained for different lengths of time depending on their interactions with its surface sites, the constituents travel at different apparent velocities in the mobile fluid, causing them to separate. The separation is based on the differential partitioning between the mobile and the stationary phases. Subtle differences in a compound's partition coefficient result in differential retention on the stationary phase and thus affect the separation.
The isoelectric point (pI, pH(I), IEP), is the pH at which a molecule carries no net electrical charge or is electrically neutral in the statistical mean. The standard nomenclature to represent the isoelectric point is pH(I). However, pI is also used. For brevity, this article uses pI. The net charge on the molecule is affected by pH of its surrounding environment and can become more positively or negatively charged due to the gain or loss, respectively, of protons (H+).

Southern blot is a method used for detection and quantification of a specific DNA sequence in DNA samples. This method is used in molecular biology. Briefly, purified DNA from a biological sample is digested with restriction enzymes, and the resulting DNA fragments are separated by using an electric current to move them through a sieve-like gel or matrix, which allows smaller fragments to move faster than larger fragments. The DNA fragments are transferred out of the gel or matrix onto a solid membrane, which is then exposed to a DNA probe labeled with a radioactive, fluorescent, or chemical tag. The tag allows any DNA fragments containing complementary sequences with the DNA probe sequence to be visualized within the Southern blot.
Protein purification is a series of processes intended to isolate one or a few proteins from a complex mixture, usually cells, tissues or whole organisms. Protein purification is vital for the specification of the function, structure and interactions of the protein of interest. The purification process may separate the protein and non-protein parts of the mixture, and finally separate the desired protein from all other proteins. Ideally, to study a protein of interest, it must be separated from other components of the cell so that contaminants will not interfere in the examination of the protein of interest's structure and function. Separation of one protein from all others is typically the most laborious aspect of protein purification. Separation steps usually exploit differences in protein size, physico-chemical properties, binding affinity and biological activity. The pure result may be termed protein isolate.
Salting out is a purification technique that utilizes the reduced solubility of certain molecules in a solution of very high ionic strength. Salting out is typically used to precipitate large biomolecules, such as proteins or DNA. Because the salt concentration needed for a given protein to precipitate out of the solution differs from protein to protein, a specific salt concentration can be used to precipitate a target protein. This process is also used to concentrate dilute solutions of proteins. Dialysis can be used to remove the salt if needed.
A chaotropic agent is a molecule in water solution that can disrupt the hydrogen bonding network between water molecules. This has an effect on the stability of the native state of other molecules in the solution, mainly macromolecules by weakening the hydrophobic effect. For example, a chaotropic agent reduces the amount of order in the structure of a protein formed by water molecules, both in the bulk and the hydration shells around hydrophobic amino acids, and may cause its denaturation.
The first isolation of deoxyribonucleic acid (DNA) was done in 1869 by Friedrich Miescher. DNA extraction is the process of isolating DNA from the cells of an organism isolated from a sample, typically a biological sample such as blood, saliva, or tissue. It involves breaking open the cells, removing proteins and other contaminants, and purifying the DNA so that it is free of other cellular components. The purified DNA can then be used for downstream applications such as PCR, sequencing, or cloning. Currently, it is a routine procedure in molecular biology or forensic analyses.
Ammonium sulfate precipitation is one of the most commonly used methods for large and laboratory scale protein purification and fractionation that can be used to separate proteins by altering their solubility in the presence of a high salt concentration.
Capillary electrophoresis (CE) is a family of electrokinetic separation methods performed in submillimeter diameter capillaries and in micro- and nanofluidic channels. Very often, CE refers to capillary zone electrophoresis (CZE), but other electrophoretic techniques including capillary gel electrophoresis (CGE), capillary isoelectric focusing (CIEF), capillary isotachophoresis and micellar electrokinetic chromatography (MEKC) belong also to this class of methods. In CE methods, analytes migrate through electrolyte solutions under the influence of an electric field. Analytes can be separated according to ionic mobility and/or partitioning into an alternate phase via non-covalent interactions. Additionally, analytes may be concentrated or "focused" by means of gradients in conductivity and pH.
Affinity chromatography is a method of separating a biomolecule from a mixture, based on a highly specific macromolecular binding interaction between the biomolecule and another substance. The specific type of binding interaction depends on the biomolecule of interest; antigen and antibody, enzyme and substrate, receptor and ligand, or protein and nucleic acid binding interactions are frequently exploited for isolation of various biomolecules. Affinity chromatography is useful for its high selectivity and resolution of separation, compared to other chromatographic methods.

Ion chromatography is a form of chromatography that separates ions and ionizable polar molecules based on their affinity to the ion exchanger. It works on almost any kind of charged molecule—including small inorganic anions, large proteins, small nucleotides, and amino acids. However, ion chromatography must be done in conditions that are one pH unit away from the isoelectric point of a protein.

Solid-phase extraction (SPE) is a solid-liquid extractive technique, by which compounds that are dissolved or suspended in a liquid mixture are separated, isolated or purified, from other compounds in this mixture, according to their physical and chemical properties. Analytical laboratories use solid phase extraction to concentrate and purify samples for analysis. Solid phase extraction can be used to isolate analytes of interest from a wide variety of matrices, including urine, blood, water, beverages, soil, and animal tissue.

A plasmid preparation is a method of DNA extraction and purification for plasmid DNA, it is an important step in many molecular biology experiments and is essential for the successful use of plasmids in research and biotechnology. Many methods have been developed to purify plasmid DNA from bacteria. During the purification procedure, the plasmid DNA is often separated from contaminating proteins and genomic DNA.
Ethanol precipitation is a method used to purify and/or concentrate RNA, DNA, and polysaccharides such as pectin and xyloglucan from aqueous solutions by adding ethanol as an antisolvent.

Acid guanidinium thiocyanate-phenol-chloroform extraction is a liquid–liquid extraction technique in biochemistry. It is widely used in molecular biology for isolating RNA. This method may take longer than a column-based system such as the silica-based purification, but has higher purity and the advantage of high recovery of RNA: an RNA column is typically unsuitable for purification of short RNA species, such as siRNA, miRNA, gRNA and tRNA.
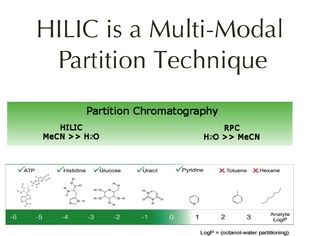
Hydrophilic interaction chromatography is a variant of normal phase liquid chromatography that partly overlaps with other chromatographic applications such as ion chromatography and reversed phase liquid chromatography. HILIC uses hydrophilic stationary phases with reversed-phase type eluents. The name was suggested by Andrew Alpert in his 1990 paper on the subject. He described the chromatographic mechanism for it as liquid-liquid partition chromatography where analytes elute in order of increasing polarity, a conclusion supported by a review and re-evaluation of published data.

Spin column-based nucleic acid purification is a solid phase extraction method to quickly purify nucleic acids. This method relies on the fact that nucleic acid will bind to the solid phase of silica under certain conditions.
Boom method is a solid phase extraction method for isolating nucleic acid from a biological sample. This method is characterized by "absorbing the nucleic acids (NA) to the silica beads".
Anion-exchange chromatography is a process that separates substances based on their charges using an ion-exchange resin containing positively charged groups, such as diethyl-aminoethyl groups (DEAE). In solution, the resin is coated with positively charged counter-ions (cations). Anion exchange resins will bind to negatively charged molecules, displacing the counter-ion. Anion exchange chromatography is commonly used to purify proteins, amino acids, sugars/carbohydrates and other acidic substances with a negative charge at higher pH levels. The tightness of the binding between the substance and the resin is based on the strength of the negative charge of the substance.
Solid-phase reversible immobilization, or SPRI, is a method of purifying nucleic acids from solution. It uses silica- or carboxyl-coated paramagnetic beads, which reversibly bind to nucleic acids in the presence of polyethylene glycol and a salt. A common application of SPRI technology is purifying samples of DNA amplified by PCR for sequencing reactions:.






