
Leucine (symbol Leu or L) is an essential amino acid that is used in the biosynthesis of proteins. Leucine is an α-amino acid, meaning it contains an α-amino group (which is in the protonated −NH3+ form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain isobutyl group, making it a non-polar aliphatic amino acid. It is essential in humans, meaning the body cannot synthesize it: it must be obtained from the diet. Human dietary sources are foods that contain protein, such as meats, dairy products, soy products, and beans and other legumes. It is encoded by the codons UUA, UUG, CUU, CUC, CUA, and CUG.

Sirolimus, also known as rapamycin and sold under the brand name Rapamune among others, is a macrolide compound that is used to coat coronary stents, prevent organ transplant rejection, treat a rare lung disease called lymphangioleiomyomatosis, and treat perivascular epithelioid cell tumor (PEComa). It has immunosuppressant functions in humans and is especially useful in preventing the rejection of kidney transplants. It is a mechanistic target of rapamycin kinase (mTOR) inhibitor that inhibits activation of T cells and B cells by reducing their sensitivity to interleukin-2 (IL-2).

The mammalian target of rapamycin (mTOR), also referred to as the mechanistic target of rapamycin, and sometimes called FK506-binding protein 12-rapamycin-associated protein 1 (FRAP1), is a kinase that in humans is encoded by the MTOR gene. mTOR is a member of the phosphatidylinositol 3-kinase-related kinase family of protein kinases.

The tumor suppressor gene FLCN encodes the protein folliculin, also known as Birt–Hogg–Dubé syndrome protein, which functions as an inhibitor of Lactate Dehydrogenase-A and a regulator of the Warburg effect. Folliculin (FLCN) is also associated with Birt–Hogg–Dubé syndrome, which is an autosomal dominant inherited cancer syndrome in which affected individuals are at risk for the development of benign cutaneous tumors (folliculomas), pulmonary cysts, and kidney tumors.
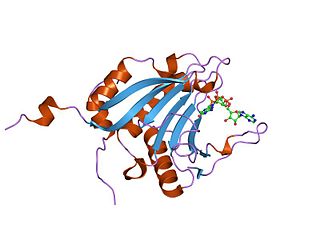
Eukaryotic translation initiation factor 4E-binding protein 1 is a protein that in humans is encoded by the EIF4EBP1 gene. inhibits cap-dependent translation by binding to translation initiation factor eIF4E. Phosphorylation of 4E-BP1 results in its release from eIF4E, thereby allows cap-dependent translation to continue thereby increasing the rate of protein synthesis.
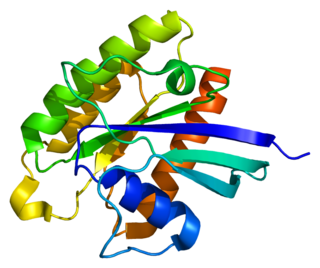
RHEB also known as Ras homolog enriched in brain (RHEB) is a GTP-binding protein that is ubiquitously expressed in humans and other mammals. The protein is largely involved in the mTOR pathway and the regulation of the cell cycle.

Ribosomal protein S6 kinase beta-1 (S6K1), also known as p70S6 kinase, is an enzyme that in humans is encoded by the RPS6KB1 gene. It is a serine/threonine kinase that acts downstream of PIP3 and phosphoinositide-dependent kinase-1 in the PI3 kinase pathway. As the name suggests, its target substrate is the S6 ribosomal protein. Phosphorylation of S6 induces protein synthesis at the ribosome.

Regulatory-associated protein of mTOR also known as raptor or KIAA1303 is an adapter protein that is encoded in humans by the RPTOR gene. Two mRNAs from the gene have been identified that encode proteins of 1335 and 1177 amino acids long.

Lipin-1 is a protein that in humans is encoded by the LPIN1 gene.

Rapamycin-insensitive companion of mammalian target of rapamycin (RICTOR) is a protein that in humans is encoded by the RICTOR gene.

DEP domain-containing mTOR-interacting protein (DEPTOR) also known as DEP domain-containing protein 6 (DEPDC6) is a protein that in humans is encoded by the DEPTOR gene.

Target of rapamycin complex subunit LST8, also known as mammalian lethal with SEC13 protein 8 (mLST8) or TORC subunit LST8 or G protein beta subunit-like, is a protein that in humans is encoded by the MLST8 gene. It is a subunit of both mTORC1 and mTORC2, complexes that regulate cell growth and survival in response to nutrient, energy, redox, and hormonal signals. It is upregulated in several human colon and prostate cancer cell lines and tissues. Knockdown of mLST8 prevented mTORC formation and inhibited tumor growth and invasiveness.

mTOR inhibitors are a class of drugs that inhibit the mammalian target of rapamycin (mTOR), which is a serine/threonine-specific protein kinase that belongs to the family of phosphatidylinositol-3 kinase (PI3K) related kinases (PIKKs). mTOR regulates cellular metabolism, growth, and proliferation by forming and signaling through two protein complexes, mTORC1 and mTORC2. The most established mTOR inhibitors are so-called rapalogs, which have shown tumor responses in clinical trials against various tumor types.

mTORC1, also known as mammalian target of rapamycin complex 1 or mechanistic target of rapamycin complex 1, is a protein complex that functions as a nutrient/energy/redox sensor and controls protein synthesis.
mTOR Complex 2 (mTORC2) is an acutely rapamycin-insensitive protein complex formed by serine/threonine kinase mTOR that regulates cell proliferation and survival, cell migration and cytoskeletal remodeling. The complex itself is rather large, consisting of seven protein subunits. The catalytic mTOR subunit, DEP domain containing mTOR-interacting protein (DEPTOR), mammalian lethal with sec-13 protein 8, and TTI1/TEL2 complex are shared by both mTORC2 and mTORC1. Rapamycin-insensitive companion of mTOR (RICTOR), mammalian stress-activated protein kinase interacting protein 1 (mSIN1), and protein observed with rictor 1 and 2 (Protor1/2) can only be found in mTORC2. Rictor has been shown to be the scaffold protein for substrate binding to mTORC2.

Michael Nip Hall is an American-Swiss molecular biologist and professor at the Biozentrum of the University of Basel, Switzerland. He discovered TOR, a protein central for regulating cell growth.

Nitrogen permease regulator-like 3 is a protein that in humans is encoded by the NPRL3 gene.

The Ragulator-Rag complex is a regulator of lysosomal signalling and trafficking in eukaryotic cells, which plays an important role in regulating cell metabolism and growth in response to nutrient availability in the cell. The Ragulator-Rag Complex is composed of five LAMTOR subunits, which work to regulate MAPK and mTOR complex 1. The LAMTOR subunits form a complex with Rag GTPase and v-ATPase, which sits on the cell’s lysosomes and detects the availability of amino acids. If the Ragulator complex receives signals for low amino acid count, it will start the process of catabolizing the cell. If there is an abundance of amino acids available to the cell, the Ragulator complex will signal that the cell can continue to grow. Ragulator proteins come in two different forms: Rag A/Rag B and Rag C/Rag D. These interact to form heterodimers with one another.

Joseph Heitman is an American physician-scientist focused on research in genetics, microbiology, and infectious diseases. He is the James B. Duke Professor and Chair of the Department of Molecular Genetics and Microbiology at Duke University School of Medicine.
Phosphatidylinositol 3-phosphate-binding protein 2 (Pib2) is a yeast protein involved in the regulation of TORC1 signaling and lysosomal membrane permeabilization. It is essential for the reactivation of TORC1 following exposure to rapamycin or nutrient starvation.

















