Related Research Articles

Rhenium is a chemical element with the symbol Re and atomic number 75. It is a silvery-gray, heavy, third-row transition metal in group 7 of the periodic table. With an estimated average concentration of 1 part per billion (ppb), rhenium is one of the rarest elements in the Earth's crust. Rhenium has the third-highest melting point and second-highest boiling point of any element at 5869 K. Rhenium resembles manganese and technetium chemically and is mainly obtained as a by-product of the extraction and refinement of molybdenum and copper ores. Rhenium shows in its compounds a wide variety of oxidation states ranging from −1 to +7.

John Kerr FRS was a Scottish physicist and a pioneer in the field of electro-optics. He is best known for the discovery of what is now called the Kerr effect.

Group 7, numbered by IUPAC nomenclature, is a group of elements in the periodic table. They are manganese (Mn), technetium (Tc), rhenium (Re), and bohrium (Bh). All known elements of group 7 are transition metals.
The year 1950 in science and technology included some significant events.

Nebulium was a proposed element found in astronomical observation of a nebula by William Huggins in 1864. The strong green emission lines of the Cat's Eye Nebula, discovered using spectroscopy, led to the postulation that an as yet unknown element was responsible for this emission. In 1927, Ira Sprague Bowen showed that the lines are emitted by doubly ionized oxygen (O2+), and no new element was necessary to explain them.

Photosystem II is the first protein complex in the light-dependent reactions of oxygenic photosynthesis. It is located in the thylakoid membrane of plants, algae, and cyanobacteria. Within the photosystem, enzymes capture photons of light to energize electrons that are then transferred through a variety of coenzymes and cofactors to reduce plastoquinone to plastoquinol. The energized electrons are replaced by oxidizing water to form hydrogen ions and molecular oxygen.
The Siegbahn notation is used in X-ray spectroscopy to name the spectral lines that are characteristic to elements. It was introduced by Manne Siegbahn.
This is a timeline of subatomic particle discoveries, including all particles thus far discovered which appear to be elementary given the best available evidence. It also includes the discovery of composite particles and antiparticles that were of particular historical importance.
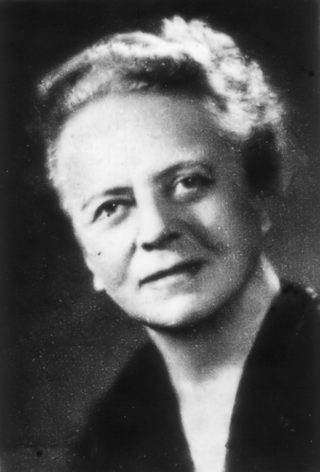
Ida Noddack, néeTacke, was a German chemist and physicist. In 1934 she was the first to mention the idea later named nuclear fission. With her husband Walter Noddack, and Otto Berg, she discovered element 75, rhenium. She was nominated three times for the Nobel Prize in Chemistry.

Rheniite is a very rare rhenium sulfide mineral with the chemical formula. It forms metallic, silver grey platey crystals in the triclinic - pinacoidal class. It has a specific gravity of 7.5.

The cyclol hypothesis is the now discredited first structural model of a folded, globular protein, formulated in the 1930s. It was based on the cyclol reaction of peptide bonds proposed by physicist Frederick Frank in 1936, in which two peptide groups are chemically crosslinked. These crosslinks are covalent analogs of the non-covalent hydrogen bonds between peptide groups and have been observed in rare cases, such as the ergopeptides.
Edmund Clifton Stoner FRS was a British theoretical physicist. He is principally known for his work on the origin and nature of itinerant ferromagnetism, including the collective electron theory of ferromagnetism and the Stoner criterion for ferromagnetism. Stoner made significant contributions to the electron configurations in the periodic table.
The Pickering series (also known as the Pickering–Fowler series) consists of three lines of singly ionized helium found, usually in absorption, in the spectra of hot stars like Wolf–Rayet stars. The name comes from Edward Charles Pickering and Alfred Fowler. The lines are produced by transitions from a higher energy level of an electron to a level with principal quantum number n = 4. The lines have wavelengths:

The Ronald Fisher bibliography contains the works published by the English statistician and biologist Ronald Fisher (1890–1962).

Rhenium(VI) chloride is the inorganic compound with the formula ReCl6. It is a black paramagnetic solid. The molecules adopt an octahedral structure as seen in tungsten(VI) chloride.
George Pirie was a Scottish mathematician, mathematical scientist, and Reverend in the Church of Scotland. He was an expert in the field of dynamics and the approximation of π.

Nuclear fission was discovered in December 1938 by chemists Otto Hahn and Fritz Strassmann and physicists Lise Meitner and Otto Robert Frisch. Fission is a nuclear reaction or radioactive decay process in which the nucleus of an atom splits into two or more smaller, lighter nuclei and often other particles. The fission process often produces gamma rays and releases a very large amount of energy, even by the energetic standards of radioactive decay. Scientists already knew about alpha decay and beta decay, but fission assumed great importance because the discovery that a nuclear chain reaction was possible led to the development of nuclear power and nuclear weapons. Hahn was awarded the 1944 Nobel Prize in Chemistry for the discovery of nuclear fission.

Sir Edmund Taylor Whittaker was a British mathematician, physicist, historian of science, and philosopher who authored three titles that remain in circulation over a century after their initial publications. His bibliography includes several books and over one hundred published papers on a variety of subjects, including mathematics, astronomy, mathematical physics, theoretical physics, philosophy, and theism. Whittaker's bibliography in the Biographical Memoirs of Fellows of the Royal Society categorises his publications into three categories: books and monographs, maths and physics articles, and biographical articles; the bibliography excludes works published in popular magazines like Scientific American. The bibliography includes eleven total books and monographs, fifty-six maths and physics articles, thirty-five philosophy and history articles, and twenty-one biographical articles. In the bibliography compiled by William Hunter McCrea in 1957, there are thirteen books and monographs and the same journal articles; McCrea counts all three volumes of A History of the Theories of Aether and Electricity as separate books and excludes the same papers. Whittaker's contributions to Scientific American include two book reviews and a popular article on mathematics.

Max Born was a widely influential German physicist and mathematician who was awarded the 1954 Nobel Prize in Physics for his pivotal role in the development of quantum mechanics. Born won the prize primarily for his contributions to the statistical interpretation of the wave function, though he is known for his work in several areas of quantum mechanics as well as solid-state physics, optics, and special relativity. Born's entry in the Biographical Memoirs of Fellows of the Royal Society included thirty books and 330 papers.
References
- ↑ Kern, Serge (1877). "On a new metal, davyum". Philosophical Magazine. Series 5. 4 (23): 158–159. doi:10.1080/14786447708639315.
- ↑ Kern, Serge (1877). "On a new metal, davyum". Philosophical Magazine. Series 5. 4 (26): 395–396. doi:10.1080/14786447708639360.
- ↑ "Davyum1". Nature. 17 (430): 245–246. 1878. Bibcode:1878Natur..17..245.. doi: 10.1038/017245a0 .
- ↑ Swjaginzew, O.; Korsunski, M.; Seljakow, N. (1927). "Dwimangan in Platinerzen". Zeitschrift für Angewandte Chemie. 40 (9): 256. Bibcode:1927AngCh..40..256S. doi:10.1002/ange.19270400905.
- ↑ Friend, J. Newton; Druce, J. G. F. (1950). "Davyum, a Possible Precursor of Rhenium (Element 75)". Nature. 165 (4203): 819. Bibcode:1950Natur.165..819F. doi: 10.1038/165819a0 . PMID 15423460. S2CID 1276756.