
A polyatomic ion is a covalent bonded set of two or more atoms, or of a metal complex, that can be considered to behave as a single unit and that has a net charge that is not zero. The term molecule may or may not be used to refer to a polyatomic ion, depending on the definition used. The prefix poly- carries the meaning "many" in Greek, but even ions of two atoms are commonly described as polyatomic.
A borate is any of a range of boron oxyanions, anions containing boron and oxygen, such as orthoborate BO3−3, metaborate BO−2, or tetraborate B4O2−7; or any salt of such anions, such as sodium metaborate, Na+[BO2]− and borax (Na+)2[B4O7]2−. The name also refers to esters of such anions, such as trimethyl borate B(OCH3)3.
An oxyanion, or oxoanion, is an ion with the generic formula A
xOz−
y. Oxyanions are formed by a large majority of the chemical elements. The formulae of simple oxyanions are determined by the octet rule. The corresponding oxyacid of an oxyanion is the compound H
zA
xO
y. The structures of condensed oxyanions can be rationalized in terms of AOn polyhedral units with sharing of corners or edges between polyhedra. The oxyanions adenosine monophosphate (AMP), adenosine diphosphate (ADP) and adenosine triphosphate (ATP) are important in biology.

In chemistry, a polyoxometalate is a polyatomic ion, usually an anion, that consists of three or more transition metal oxyanions linked together by shared oxygen atoms to form closed 3-dimensional frameworks. The metal atoms are usually group 6 or less commonly group 5 and group 7 transition metals in their high oxidation states. Polyoxometalates are often colorless, orange or red diamagnetic anions. Two broad families are recognized, isopolymetalates, composed of only one kind of metal and oxide, and heteropolymetalates, composed of one metal, oxide, and a main group oxyanion. Many exceptions to these general statements exist.
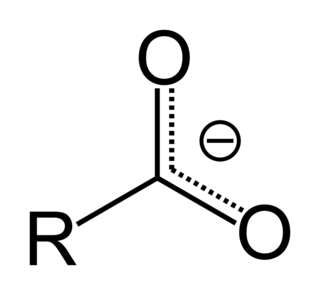
In organic chemistry, a carboxylate is the conjugate base of a carboxylic acid, RCOO−. It is an ion with negative charge.

An iodate is the polyatomic anion with the formula IO−3. It is the most common form of iodine in nature, as it comprises the major iodine-containing ores. Iodate salts are often colorless. They are the salts of iodic acid.
In chemistry, the perbromate ion is the anion having the chemical formula BrO−
4. It is an oxyanion of bromine, the conjugate base of perbromic acid, in which bromine has the oxidation state +7. Unlike its chlorine and iodine analogs, it is difficult to synthesize. It has tetrahedral molecular geometry.

In chemistry tellurate is a compound containing an oxyanion of tellurium where tellurium has an oxidation number of +6. In the naming of inorganic compounds it is a suffix that indicates a polyatomic anion with a central tellurium atom.
In chemistry, an arsenite is a chemical compound containing an arsenic oxyanion where arsenic has oxidation state +3. Note that in fields that commonly deal with groundwater chemistry, arsenite is used generically to identify soluble AsIII anions. IUPAC have recommended that arsenite compounds are to be named as arsenate(III), for example ortho-arsenite is called trioxidoarsenate(III). Ortho-arsenite contrasts to the corresponding anions of the lighter members of group 15, phosphite which has the structure HPO2−3 and nitrite, NO−2 which is bent.

In chemistry, the heteropolymetalates are a subset of the polyoxometalates, which consist of three or more transition metal oxyanions linked together by shared oxygen atoms to form a closed 3-dimensional molecular framework. In contrast to isopolymetalates, which contain only one kind of metal atom, the heteropolymetalates contain differing main group oxyanions. The metal atoms are usually group 6 or less commonly group 5 transition metals in their highest oxidation states. They are usually colorless to orange, diamagnetic anions. For most heteropolymetalates the W, Mo, or V, is complemented by main group oxyanions phosphate and silicate. Many exceptions to these general statements exist, and the class of compounds includes hundreds of examples.
In chemical nomenclature, the IUPAC nomenclature of inorganic chemistry is a systematic method of naming inorganic chemical compounds, as recommended by the International Union of Pure and Applied Chemistry (IUPAC). It is published in Nomenclature of Inorganic Chemistry. Ideally, every inorganic compound should have a name from which an unambiguous formula can be determined. There is also an IUPAC nomenclature of organic chemistry.

Phosphotungstic acid (PTA) or tungstophosphoric acid (TPA), is a heteropoly acid with the chemical formula H3PW12O40]. It forms hydrates H3[PW12O40]·nH2O. It is normally isolated as the n = 24 hydrate but can be desiccated to the hexahydrate (n = 6). EPTA is the name of ethanolic phosphotungstic acid, its alcohol solution used in biology. It has the appearance of small, colorless-grayish or slightly yellow-green crystals, with melting point 89 °C (24 H2O hydrate). It is odorless and soluble in water (200 g/100 ml). It is not especially toxic, but is a mild acidic irritant. The compound is known by a variety of names and acronyms (see 'other names' section of infobox).

Chromium is a member of group 6, of the transition metals. The +3 and +6 states occur most commonly within chromium compounds, followed by +2; charges of +1, +4 and +5 for chromium are rare, but do nevertheless occasionally exist.

The tetrathionate anion, S
4O2−
6, is a sulfur oxyanion derived from the compound tetrathionic acid, H2S4O6. Two of the sulfur atoms present in the ion are in oxidation state 0 and two are in oxidation state +5. Alternatively, the compound can be viewed as the adduct resulting from the binding of S2−
2 to SO3. Tetrathionate is one of the polythionates, a family of anions with the formula [Sn(SO3)2]2−. Its IUPAC name is 2-(dithioperoxy)disulfate, and the name of its corresponding acid is 2-(dithioperoxy)disulfuric acid. The Chemical Abstracts Service identifies tetrathionate by the CAS Number 15536-54-6.
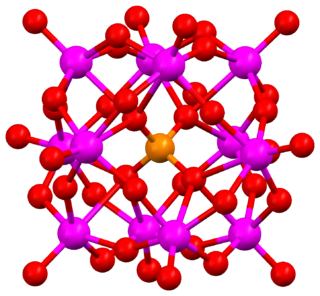
The Keggin structure is the best known structural form for heteropoly acids. It is the structural form of α-Keggin anions, which have a general formula of [XM12O40]n−, where X is the heteroatom, M is the addendum atom, and O represents oxygen. The structure self-assembles in acidic aqueous solution and is a commonly used type of polyoxometalate catalysts.

Molybdenum blue is a term applied to:
In chemistry, an ate complex is a salt formed by the reaction of a Lewis acid with a Lewis base whereby the central atom increases its valence and gains a negative formal charge..
Thiophosphates (or phosphorothioates, PS) are chemical compounds and anions with the general chemical formula PS
4−xO3−
x (x = 0, 1, 2, or 3) and related derivatives where organic groups are attached to one or more O or S. Thiophosphates feature tetrahedral phosphorus(V) centers.
Orthonitrate is a tetrahedral oxyanion of nitrogen with the formula NO3−
4. It was first identified in 1977 and is currently known in only two compounds, sodium orthonitrate (Na3NO4) and potassium orthonitrate (K3NO4). The corresponding oxoacid, orthonitric acid (H3NO4), is hypothetical and has never been observed. Sodium and potassium orthonitrate can be prepared by fusion of the nitrate and metal oxide under high temperatures and ideally high pressures (several GPa).
The borotellurates are heteropoly anion compounds which have tellurate groups attached to boron atoms. The ratio of tellurate to borate reflects the degree of condensation. In [TeO4(BO3)2]8- the anions are linked into a chain. In [TeO2(BO3)4]10− the structure is zero dimensional with isolated anions. These arrangements of oxygen around boron and tellurium can have forms resembling silicates. The first borotellurates to be discovered were the mixed sodium rare earth compounds in 2015.












