
Alkaloids are a class of basic, naturally occurring organic compounds that contain at least one nitrogen atom. This group also includes some related compounds with neutral and even weakly acidic properties. Some synthetic compounds of similar structure may also be termed alkaloids. In addition to carbon, hydrogen and nitrogen, alkaloids may also contain oxygen or sulfur. More rarely still, they may contain elements such as phosphorus, chlorine, and bromine.

Aconitum, also known as aconite, monkshood, wolfsbane, leopard's bane, devil's helmet or blue rocket, is a genus of over 250 species of flowering plants belonging to the family Ranunculaceae. These herbaceous perennial plants are chiefly native to the mountainous parts of the Northern Hemisphere in North America, Europe, and Asia; growing in the moisture-retentive but well-draining soils of mountain meadows.

Aconitine is an alkaloid toxin produced by various plant species belonging to the genus Aconitum, known also commonly by the names wolfsbane and monkshood. Monkshood is notorious for its toxic properties.

Ranunculaceae is a family of over 2,000 known species of flowering plants in 43 genera, distributed worldwide.

Delphinium is a genus of about 300 species of annual and perennial flowering plants in the family Ranunculaceae, native throughout the Northern Hemisphere and also on the high mountains of tropical Africa. The genus was erected by Carl Linnaeus.

Consolida is a genus of about 40 species of annual flowering plants in the family Ranunculaceae, native to western Europe, the Mediterranean and Asia. Phylogenetic studies show that Consolida is actually an annual clade nested within the genus Delphinium and it has been treated as a synonym of Delphinium in Kew's Plants of the World Online. The name of the genus comes from an archaic use of consolidation, meaning "healing", in reference to the plant's medieval use for healing wounds.

Pseudaconitine, also known as nepaline (C36H51NO12), is an extremely toxic alkaloid found in high quantities in the roots of Aconitum ferox, also known as Indian Monkshood, which belongs to the family Ranunculaceae. The plant is found in East Asia, including the Himalayas.

Consolida regalis, known as forking larkspur, rocket-larkspur, and field larkspur, is an annual herbaceous plant belonging to the genus Consolida of the buttercup family (Ranunculaceae).

Consolida ajacis is an annual flowering plant of the family Ranunculaceae native to Eurasia. It is widespread in other areas, including much of North America, where it is an introduced species. It is frequently grown in gardens as an ornamental for its spikes of blue, pink or white flowers. It may reach a meter in height. Since the aerial parts and seeds of C. ajacis have been found to contain diterpenoid alkaloids, including the highly toxic methyllycaconitine, the plants should be considered as poisonous.

Aconitum lycoctonum is a species of flowering plant in the genus Aconitum, of the family Ranunculaceae, native to much of Europe and northern Asia. It is found in lowlands to the subalpine zone, mainly in forests and shaded habitats. Along with A. napellus, A. lycoctonum is of the most common European species of the Aconitum genus. They are also grown ornamentally in gardens, thriving well in ordinary garden soil. As such, A. lycoctonum can be found in North America, especially in eastern Canada, often in old gardens or as garden escapees.

Methyllycaconitine (MLA) is a diterpenoid alkaloid found in many species of Delphinium (larkspurs). In common with many other diterpenoid alkaloids, it is toxic to animals, although the acute toxicity varies with species. Early research was focused on identifying, and characterizing the properties of methyllycaconitine as one of the principal toxins in larkspurs responsible for livestock poisoning in the mountain rangelands of North America. Methyllycaconitine has been explored as a possible therapeutic agent for the treatment of spastic paralysis, and it has been shown to have insecticidal properties. Most recently, it has become an important molecular probe for studying the pharmacology of the nicotinic acetylcholine receptor.

Staphisagria macrosperma, formerly known as Delphinium staphisagria, is a species of Staphisagria of the family Ranunculaceae. It used to belong to the subgenus or section Staphisagria of the genus Delphinium, but molecular evidence suggests Staphisagria should be a genus which is a sister group to the Aconitum-Delphinium clade. It is described botanically as a stoutly-stemmed, hairy biennial with large palmate leaves up to 6 inches (15 cm) across. The flowers are mauve-blue to blue, short-spurred, and up to 1 inch (2.5 cm) across, occurring in racemes. The plant grows to a height of 4–5 feet. It grows throughout the Mediterranean. All parts of this plant are highly toxic and should not be ingested in any quantity.

Aconitum napellus, monkshood, aconite, Venus' chariot or wolfsbane, is a species of highly toxic flowering plants in the genus Aconitum of the family Ranunculaceae, native and endemic to western and central Europe. It is an herbaceous perennial plant growing to 1 m tall, with hairless stems and leaves. The leaves are rounded, 5–10 cm (2.0–3.9 in) diameter, palmately divided into five to seven deeply lobed segments. The flowers are dark purple to bluish-purple, narrow oblong helmet-shaped, 1–2 cm (0.39–0.79 in) tall. Plants native to Asia and North America formerly listed as A. napellus are now regarded as separate species. The plant is extremely poisonous in both ingestion and body contact.

Delphinine is a toxic diterpenoid alkaloid found in plants from the Delphinium (larkspur) and Atragene genera, both in the family Ranunculaceae. Delphinine is the principal alkaloid found in Delphinium staphisagria seeds – at one time, under the name stavesacre, a very well known herbal treatment for body lice. It is related in structure and has similar effects to aconitine, acting as an allosteric modulator of voltage gated sodium channels, and producing low blood pressure, slowed heart rate and abnormal heart rhythms. These effects make it highly poisonous. While it has been used in some alternative medicines, most of the medical community does not recommend using it due to its extreme toxicity.
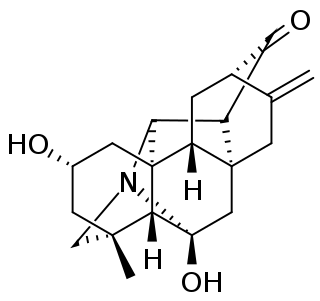
Panicudine (6-hydroxy-11-deoxy-13-dehydrohetisane) is a C20-diterpene alkaloid of the hetisine type, first isolated from Aconitum paniculatum. It has empirical formula C20H25NO3 and a melting point of 249–250 °C. The structure was determined to be a hetisine type diterpene by noting infrared spectrum absorption bands of 3405 cm−1 (OH), 1718 (C=O), and 1650 (C=C), a proton magnetic resonance spectrum with "secondary hydroxy (4.02 ppm, m, 1H, W1/2 = 10 Hz), exomethylene (4.87 and 4.76 ppm, br.s, 1H each), and tertiary methyl (1.29 ppm, s, 3H) groups and the absence of N-methyl, N-ethyl, and methoxy groups." Additional ultraviolet spectrum and carbon-13 NMR data, confirmed by high resolution mass spectrometry, completed the determination of the structure.

Akuammicine is a monoterpene indole alkaloid of the Vinca sub-group. It is found in the Apocynaceae family of plants including Picralima nitida, Vinca minor and the Aspidosperma.

Gigactonine is a naturally occurring diterpene alkaloid first isolated from Aconitum gigas. It occurs widely in the Ranunculaceae plant family. The polycyclic ring system of this chemical compound contains nineteen carbon atoms and one nitrogen atom, which is the same as in aconitine and this is reflected in its preferred IUPAC name.
Aconitum flavum is a species of flowering plant in the genus Aconitum of the family Ranunculaceae, native and endemic to northwestern Sichuan, northern Tibet, Qinghai, Gansu, southern Ningxia and southern Inner Mongolia.

Karel František Wiesner was a Canadian chemist of Czech origin known for his contributions to the chemistry of natural products, notably aconitum alkaloids and digitalis glycosides.

Staphidine is a bis-diterpene alkaloid of the atisane type, found in the tissues of Delphinium staphisagria in the larkspur family (Ranunculaceae) along with staphimine and staphinine. Similar alkaloids are found in the genus Aconitum in that family, as well as Spiraea in the Rosaceae family.




















