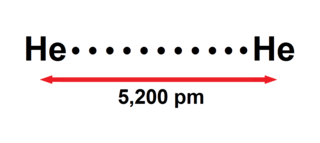Muonium is an exotic atom made up of an antimuon and an electron, which was discovered in 1960 by Vernon W. Hughes and is given the chemical symbol Mu. During the muon's 2.2 µs lifetime, muonium can undergo chemical reactions. Because, like a proton, the antimuon's mass is vastly larger than that of the electron, muonium is more similar to atomic hydrogen than positronium. Its Bohr radius and ionization energy are within 0.5% of hydrogen, deuterium, and tritium, and thus it can usefully be considered as an exotic light isotope of hydrogen.
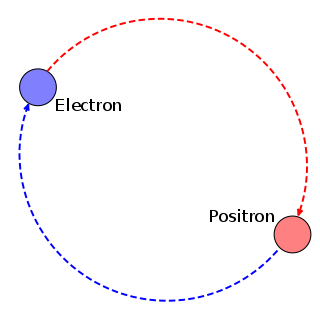
Positronium (Ps) is a system consisting of an electron and its anti-particle, a positron, bound together into an exotic atom, specifically an onium. Unlike hydrogen, the system has no protons. The system is unstable: the two particles annihilate each other to predominantly produce two or three gamma-rays, depending on the relative spin states. The energy levels of the two particles are similar to that of the hydrogen atom. However, because of the reduced mass, the frequencies of the spectral lines are less than half of those for the corresponding hydrogen lines.

Antihydrogen is the antimatter counterpart of hydrogen. Whereas the common hydrogen atom is composed of an electron and proton, the antihydrogen atom is made up of a positron and antiproton. Scientists hope that studying antihydrogen may shed light on the question of why there is more matter than antimatter in the observable universe, known as the baryon asymmetry problem. Antihydrogen is produced artificially in particle accelerators.
An exotic atom is an otherwise normal atom in which one or more sub-atomic particles have been replaced by other particles of the same charge. For example, electrons may be replaced by other negatively charged particles such as muons or pions. Because these substitute particles are usually unstable, exotic atoms typically have very short lifetimes and no exotic atom observed so far can persist under normal conditions.
The Antihydrogen Trap (ATRAP) collaboration at the Antiproton Decelerator facility at CERN, Geneva, is responsible for the AD-2 experiment. It is a continuation of the TRAP collaboration, which started taking data for the TRAP experiment in 1985. The TRAP experiment pioneered cold antiprotons, cold positrons, and first made the ingredients of cold antihydrogen to interact. Later ATRAP members pioneered accurate hydrogen spectroscopy and observed the first hot antihydrogen atoms.
Penning ionization is a form of chemi-ionization, an ionization process involving reactions between neutral atoms or molecules. The Penning effect is put to practical use in applications such as gas-discharge neon lamps and fluorescent lamps, where the lamp is filled with a penning mixture to improve the electrical characteristics of the lamps.
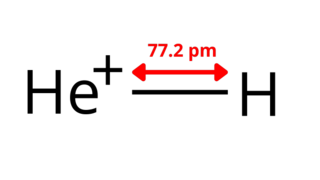
The helium hydride ion, hydridohelium(1+) ion, or helonium is a cation (positively charged ion) with chemical formula HeH+. It consists of a helium atom bonded to a hydrogen atom, with one electron removed. It can also be viewed as protonated helium. It is the lightest heteronuclear ion, and is believed to be the first compound formed in the Universe after the Big Bang.

An onium is a bound state of a particle and its antiparticle. These states are usually named by adding the suffix -onium to the name of one of the constituent particles, with one exception for "muonium"; a muon–antimuon bound pair is called "true muonium" to avoid confusion with old nomenclature.

Positronium hydride, or hydrogen positride is an exotic molecule consisting of a hydrogen atom bound to an exotic atom of positronium. Its formula is PsH. It was predicted to exist in 1951 by A Ore, and subsequently studied theoretically, but was not observed until 1990. R. Pareja, R. Gonzalez from Madrid trapped positronium in hydrogen laden magnesia crystals. The trap was prepared by Yok Chen from the Oak Ridge National Laboratory. In this experiment the positrons were thermalized so that they were not traveling at high speed, and they then reacted with H− ions in the crystal. In 1992 it was created in an experiment done by David M. Schrader and F.M. Jacobsen and others at the Aarhus University in Denmark. The researchers made the positronium hydride molecules by firing intense bursts of positrons into methane, which has the highest density of hydrogen atoms. Upon slowing down, the positrons were captured by ordinary electrons to form positronium atoms which then reacted with hydrogen atoms from the methane.

Positron annihilation spectroscopy (PAS) or sometimes specifically referred to as positron annihilation lifetime spectroscopy (PALS) is a non-destructive spectroscopy technique to study voids and defects in solids.
Giacinto Scoles is a European and North American chemist and physicist who is best known for his pioneering development of molecular beam methods for the study of weak van der Waals forces between atoms, molecules, and surfaces. He developed the cryogenic bolometer as a universal detector of atomic and molecule beams that not only can detect a small flux of molecules, but also responds to the internal energy of the molecules. This is the basis for the optothermal spectroscopy technique which Scoles and others have used to obtain very high signal-to noise and high resolution ro-vibrational spectra.
Inelastic electron tunneling spectroscopy (IETS) is an experimental tool for studying the vibrations of molecular adsorbates on metal oxides. It yields vibrational spectra of the adsorbates with high resolution (< 0.5 meV) and high sensitivity (< 1013 molecules are required to provide a spectrum). An additional advantage is the fact that optically forbidden transitions may be observed as well. Within IETS, an oxide layer with molecules adsorbed on it is put between two metal plates. A bias voltage is applied between the two contacts. An energy diagram of the metal-oxide-metal device under bias is shown in the top figure. The metal contacts are characterized by a constant density of states, filled up to the Fermi energy. The metals are assumed to be equal. The adsorbates are situated on the oxide material. They are represented by a single bridge electronic level, which is the upper dashed line. If the insulator is thin enough, there is a finite probability that the incident electron tunnels through the barrier. Since the energy of the electron is not changed by this process, it is an elastic process. This is shown in the left figure.

The dihydrogen cation or hydrogen molecular ion is a cation with formula H+
2. It consists of two hydrogen nuclei (protons), each sharing a single electron. It is the simplest molecular ion.

Calcium monohydride is a molecule composed of calcium and hydrogen with formula CaH. It can be found in stars as a gas formed when calcium atoms are present with hydrogen atoms.
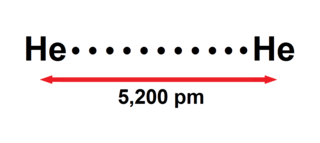
The helium dimer is a van der Waals molecule with formula He2 consisting of two helium atoms. This chemical is the largest diatomic molecule—a molecule consisting of two atoms bonded together. The bond that holds this dimer together is so weak that it will break if the molecule rotates, or vibrates too much. It can only exist at very low cryogenic temperatures.
AEgIS, AD-6, is an experiment at the Antiproton Decelerator facility at CERN. Its primary goal is to measure directly the effect of Earth's gravitational field on antihydrogen atoms with significant precision. Indirect bounds that assume the validity of, for example, the universality of free fall, the Weak Equivalence Principle or CPT symmetry also in the case of antimatter constrain an anomalous gravitational behavior to a level where only precision measurements can provide answers. Vice versa, antimatter experiments with sufficient precision are essential to validate these fundamental assumptions. AEgIS was originally proposed in 2007. Construction of the main apparatus was completed in 2012. Since 2014, two laser systems with tunable wavelengths and synchronized to the nanosecond for specific atomic excitation have been successfully commissioned.

The buffer-gas trap (BGT) is a device used to accumulate positrons efficiently while minimizing positron loss due to annihilation, which occurs when an electron and positron collide and the energy is converted to gamma rays. The BGT is used for a variety of research applications, particularly those that benefit from specially tailored positron gases, plasmas and/or pulsed beams. Examples include use of the BGT to create antihydrogen and the positronium molecule.
The rotating wall technique is a method used to compress a single-component plasma confined in an electromagnetic trap. It is one of many scientific and technological applications that rely on storing charged particles in vacuum. This technique has found extensive use in improving the quality of these traps and in tailoring of both positron and antiproton plasmas for a variety of end uses.

John Holmes Malmberg was an American plasma physicist and a professor at the University of California, San Diego. He was known for making the first experimental measurements of Landau damping of plasma waves in 1964, as well as for his research on non-neutral plasmas and the development of the Penning–Malmberg trap.

The Penning–Malmberg trap, named after Frans Penning and John Malmberg, is an electromagnetic device used to confine large numbers of charged particles of a single sign of charge. Much interest in Penning–Malmberg (PM) traps arises from the fact that if the density of particles is large and the temperature is low, the gas will become a single-component plasma. While confinement of electrically neutral plasmas is generally difficult, single-species plasmas can be confined for long times in PM traps. They are the method of choice to study a variety of plasma phenomena. They are also widely used to confine antiparticles such as positrons and antiprotons for use in studies of the properties of antimatter and interactions of antiparticles with matter.