Gel electrophoresis is a method for separation and analysis of biomacromolecules and their fragments, based on their size and charge. It is used in clinical chemistry to separate proteins by charge or size and in biochemistry and molecular biology to separate a mixed population of DNA and RNA fragments by length, to estimate the size of DNA and RNA fragments or to separate proteins by charge.

A nuclear pore is a part of a large complex of proteins, known as a nuclear pore complex that spans the nuclear envelope, which is the double membrane surrounding the eukaryotic cell nucleus. There are approximately 1,000 nuclear pore complexes (NPCs) in the nuclear envelope of a vertebrate cell, but this number varies depending on cell type and the stage in the life cycle. The human nuclear pore complex (hNPC) is a 110 megadalton (MDa) structure. The proteins that make up the nuclear pore complex are known as nucleoporins; each NPC contains at least 456 individual protein molecules and is composed of 34 distinct nucleoporin proteins. About half of the nucleoporins typically contain solenoid protein domains—either an alpha solenoid or a beta-propeller fold, or in some cases both as separate structural domains. The other half show structural characteristics typical of "natively unfolded" or intrinsically disordered proteins, i.e. they are highly flexible proteins that lack ordered tertiary structure. These disordered proteins are the FG nucleoporins, so called because their amino-acid sequence contains many phenylalanine—glycine repeats.

Kidney dialysis is the process of removing excess water, solutes, and toxins from the blood in people whose kidneys can no longer perform these functions naturally. This is referred to as renal replacement therapy. The first successful dialysis was performed in 1943.

Size-exclusion chromatography (SEC), also known as molecular sieve chromatography, is a chromatographic method in which molecules in solution are separated by their size, and in some cases molecular weight. It is usually applied to large molecules or macromolecular complexes such as proteins and industrial polymers. Typically, when an aqueous solution is used to transport the sample through the column, the technique is known as gel-filtration chromatography, versus the name gel permeation chromatography, which is used when an organic solvent is used as a mobile phase. The chromatography column is packed with fine, porous beads which are commonly composed of dextran, agarose, or polyacrylamide polymers. The pore sizes of these beads are used to estimate the dimensions of macromolecules. SEC is a widely used polymer characterization method because of its ability to provide good molar mass distribution (Mw) results for polymers.

Polyacrylamide gel electrophoresis (PAGE) is a technique widely used in biochemistry, forensic chemistry, genetics, molecular biology and biotechnology to separate biological macromolecules, usually proteins or nucleic acids, according to their electrophoretic mobility. Electrophoretic mobility is a function of the length, conformation, and charge of the molecule. Polyacrylamide gel electrophoresis is a powerful tool used to analyze RNA samples. When polyacrylamide gel is denatured after electrophoresis, it provides information on the sample composition of the RNA species.

The lipid bilayer is a thin polar membrane made of two layers of lipid molecules. These membranes are flat sheets that form a continuous barrier around all cells. The cell membranes of almost all organisms and many viruses are made of a lipid bilayer, as are the nuclear membrane surrounding the cell nucleus, and membranes of the membrane-bound organelles in the cell. The lipid bilayer is the barrier that keeps ions, proteins and other molecules where they are needed and prevents them from diffusing into areas where they should not be. Lipid bilayers are ideally suited to this role, even though they are only a few nanometers in width, because they are impermeable to most water-soluble (hydrophilic) molecules. Bilayers are particularly impermeable to ions, which allows cells to regulate salt concentrations and pH by transporting ions across their membranes using proteins called ion pumps.

Semipermeable membrane is a type of biological or synthetic, polymeric membrane that will allow certain molecules or ions to pass through it by osmosis. The rate of passage depends on the pressure, concentration, and temperature of the molecules or solutes on either side, as well as the permeability of the membrane to each solute. Depending on the membrane and the solute, permeability may depend on solute size, solubility, properties, or chemistry. How the membrane is constructed to be selective in its permeability will determine the rate and the permeability. Many natural and synthetic materials which are rather thick are also semipermeable. One example of this is the thin film on the inside of the egg.
Ultrafiltration (UF) is a variety of membrane filtration in which forces like pressure or concentration gradients lead to a separation through a semipermeable membrane. Suspended solids and solutes of high molecular weight are retained in the so-called retentate, while water and low molecular weight solutes pass through the membrane in the permeate (filtrate). This separation process is used in industry and research for purifying and concentrating macromolecular (103–106 Da) solutions, especially protein solutions.
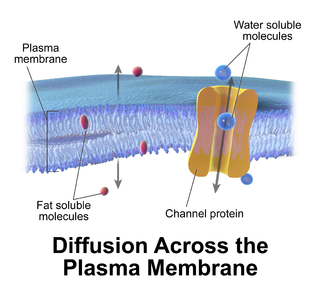
Passive transport is a type of membrane transport that does not require energy to move substances across cell membranes. Instead of using cellular energy, like active transport, passive transport relies on the second law of thermodynamics to drive the movement of substances across cell membranes. Fundamentally, substances follow Fick's first law, and move from an area of high concentration to one of low concentration because this movement increases the entropy of the overall system. The rate of passive transport depends on the permeability of the cell membrane, which, in turn, depends on the organization and characteristics of the membrane lipids and proteins. The four main kinds of passive transport are simple diffusion, facilitated diffusion, filtration, and/or osmosis.
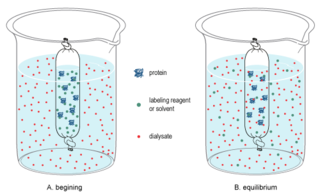
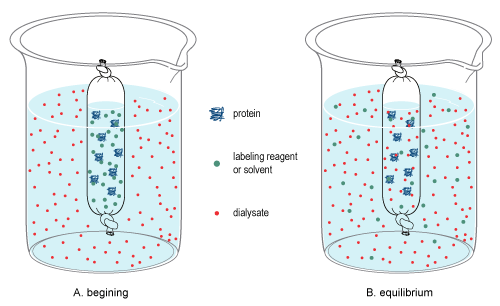
In chemistry, dialysis is the process of separating molecules in solution by the difference in their rates of diffusion through a semipermeable membrane, such as dialysis tubing.

Hemodialysis, also spelled haemodialysis, or simply dialysis, is a process of purifying the blood of a person whose kidneys are not working normally. This type of dialysis achieves the extracorporeal removal of waste products such as creatinine and urea and free water from the blood when the kidneys are in a state of kidney failure. Hemodialysis is one of three renal replacement therapies. An alternative method for extracorporeal separation of blood components such as plasma or cells is apheresis.
In cellular biology, membrane transport refers to the collection of mechanisms that regulate the passage of solutes such as ions and small molecules through biological membranes, which are lipid bilayers that contain proteins embedded in them. The regulation of passage through the membrane is due to selective membrane permeability - a characteristic of biological membranes which allows them to separate substances of distinct chemical nature. In other words, they can be permeable to certain substances but not to others.
An artificial membrane, or synthetic membrane, is a synthetically created membrane which is usually intended for separation purposes in laboratory or in industry. Synthetic membranes have been successfully used for small and large-scale industrial processes since the middle of twentieth century. A wide variety of synthetic membranes is known. They can be produced from organic materials such as polymers and liquids, as well as inorganic materials. The most of commercially utilized synthetic membranes in separation industry are made of polymeric structures. They can be classified based on their surface chemistry, bulk structure, morphology, and production method. The chemical and physical properties of synthetic membranes and separated particles as well as a choice of driving force define a particular membrane separation process. The most commonly used driving forces of a membrane process in industry are pressure and concentration gradients. The respective membrane process is therefore known as filtration. Synthetic membranes utilized in a separation process can be of different geometry and of respective flow configuration. They can also be categorized based on their application and separation regime. The best known synthetic membrane separation processes include water purification, reverse osmosis, dehydrogenation of natural gas, removal of cell particles by microfiltration and ultrafiltration, removal of microorganisms from dairy products, and Dialysis.

Gas mixtures can be effectively separated by synthetic membranes made from polymers such as polyamide or cellulose acetate, or from ceramic materials.

Ion chromatography separates ions and polar molecules based on their affinity to the ion exchanger. It works on almost any kind of charged molecule—including large proteins, small nucleotides, and amino acids. However, ion chromatography must be done in conditions that are one unit away from the isoelectric point of a protein.
Molecular weight cut-off or MWCO refers to the lowest molecular weight solute in which 90% of the solute is retained by the membrane, or the molecular weight of the molecule that is 90% retained by the membrane.
Membrane technology covers all engineering approaches for the transport of substances between two fractions with the help of semi-permeable membranes. In general, mechanical separation processes for separating gaseous or liquid streams use membrane technology.

The cell membrane is a biological membrane that separates and protects the interior of all cells from the outside environment. The cell membrane consists of a lipid bilayer, made up of two layers of phospholipids with cholesterols interspersed between them, maintaining appropriate membrane fluidity at various temperatures. The membrane also contains membrane proteins, including integral proteins that span the membrane and serve as membrane transporters, and peripheral proteins that loosely attach to the outer (peripheral) side of the cell membrane, acting as enzymes to facilitate interaction with the cell's environment. Glycolipids embedded in the outer lipid layer serve a similar purpose. The cell membrane controls the movement of substances in and out of cells and organelles, being selectively permeable to ions and organic molecules. In addition, cell membranes are involved in a variety of cellular processes such as cell adhesion, ion conductivity and cell signalling and serve as the attachment surface for several extracellular structures, including the cell wall and the carbohydrate layer called the glycocalyx, as well as the intracellular network of protein fibers called the cytoskeleton. In the field of synthetic biology, cell membranes can be artificially reassembled.
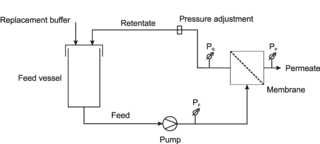
Diafiltration is a dilution process that involves removal or separation of components of a solution based on their molecular size by using micro-molecule permeable filters in order to obtain pure solution.

Desalting and buffer exchange are methods to separate soluble macromolecules from smaller molecules (desalting) or replace the buffer system used for another one suitable for a downstream application. These methods are based on gel filtration chromatography, also called molecular sieve chromatography, which is a form of size-exclusion chromatography. Desalting and buffer exchange are two of the most common gel filtration chromatography applications, and they can be performed using the same resin.