
In chemistry, an ester is a compound derived from an acid in which the hydrogen atom (H) of at least one acidic hydroxyl group of that acid is replaced by an organyl group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well, but not according to the IUPAC.
Tin(IV) chloride, also known as tin tetrachloride or stannic chloride, is an inorganic compound with the formula SnCl4. It is a colorless hygroscopic liquid, which fumes on contact with air. It is used as a precursor to other tin compounds. It was first discovered by Andreas Libavius (1550–1616) and was known as spiritus fumans libavii.

Organotin chemistry is the scientific study of the synthesis and properties of organotin compounds or stannanes, which are organometallic compounds containing tin–carbon bonds. The first organotin compound was diethyltin diiodide, discovered by Edward Frankland in 1849. The area grew rapidly in the 1900s, especially after the discovery of the Grignard reagents, which are useful for producing Sn–C bonds. The area remains rich with many applications in industry and continuing activity in the research laboratory.

Rhenium(VII) oxide is the inorganic compound with the formula Re2O7. This yellowish solid is the anhydride of HOReO3. Perrhenic acid, Re2O7·2H2O, is closely related to Re2O7. Re2O7 is the raw material for all rhenium compounds, being the volatile fraction obtained upon roasting the host ore.
Tin(II) bromide is a chemical compound of tin and bromine with a chemical formula of SnBr2. Tin is in the +2 oxidation state. The stability of tin compounds in this oxidation state is attributed to the inert pair effect.
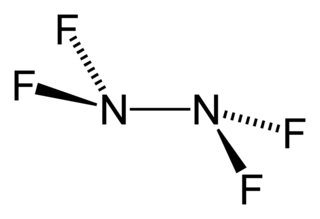
Tetrafluorohydrazine or perfluorohydrazine, N2F4, is a colourless, nonflammable, reactive inorganic gas. It is a fluorinated analog of hydrazine.

Protoporphyrin IX is an organic compound, classified as a porphyrin, that plays an important role in living organisms as a precursor to other critical compounds like heme (hemoglobin) and chlorophyll. It is a deeply colored solid that is not soluble in water. The name is often abbreviated as PPIX.

n-Butylamine is an organic compound (specifically, an amine) with the formula CH3(CH2)3NH2. This colourless liquid is one of the four isomeric amines of butane, the others being sec-butylamine, tert-butylamine, and isobutylamine. It is a liquid having the fishy, ammonia-like odor common to amines. The liquid acquires a yellow color upon storage in air. It is soluble in all organic solvents. Its vapours are heavier than air and it produces toxic oxides of nitrogen during combustion.

Trimethyltin chloride is an organotin compound with the formula (CH3)3SnCl. It is a white solid that is highly toxic and malodorous. It is susceptible to hydrolysis.

Dibutyltin oxide, or dibutyloxotin, is an organotin compound with the chemical formula (C4H9)2SnO. It is a colorless solid that, when pure, is insoluble in organic solvents. It is used as a reagent and a catalyst.

In chemistry, methanetetracarboxylate is a tetravalent anion with formula C5O4−8 or C(−CO−2)4. It has four carboxylate groups attached to a central carbon atom; so it has the same carbon backbone as neopentane. It is an oxocarbon anion, that is, consists only of carbon and oxygen.

Stannole is an organotin compound with the formula (CH)4SnH2. It is classified as a metallole, i.e. an unsaturated five-membered ring containing a heteroatom. It is a structural analog of cyclopentadiene, with tin replacing the saturated carbon atom. Substituted derivatives, which have been synthesized, are also called stannoles.
Nonadecylic acid, or nonadecanoic acid, is a 19-carbon saturated fatty acid with the chemical formula CH3(CH2)17COOH. It forms salts called nonadecylates. Nonadecylic acid can be found in fats and vegetable oils, although it is rare.

Stannoxane is a functional group in organotin chemistry with the connectivity SnIV−O−SnIV. Aside from the oxide group, usually 3 or 4 other substituents are attached to tin. In aqueous or aquatic environments, most organotin compounds contain this group.

Tetravinyltin (also known as tetravinylstannane) is an organotin compound with a chemical formula of C8H12Sn.

Cyhexatin, also known as tricyclohexyltin hydroxide is an organometallic compound of tin with the chemical formula (C6H11)3SnOH.

Dipropyltin dichloride is an organotin compound with the chemical formula (CH3CH2CH2)2SnCl2. It is a white solid. This chemical belongs to a subclass of organotin compounds called diorganotin dihalides.

S,S'-Dimethyl dithiocarbonate is an organic compound with the chemical formula OC(SCH3)2. It is a colorless liquid. It is a methyl ester of dithiocarbonic S,S-acid. It is a thioester. It is an analog of dimethyl carbonate, where the two oxygen atoms from the −OCH3 groups are replaced by sulfur atoms. In terms of the name of this thioester, it is derived from an esterification of dithiocarbonic S,S-acid with methanethiol.

















