In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula R−O−R′, where R and R′ represent the alkyl or aryl groups. Ethers can again be classified into two varieties: if the alkyl or aryl groups are the same on both sides of the oxygen atom, then it is a simple or symmetrical ether, whereas if they are different, the ethers are called mixed or unsymmetrical ethers. A typical example of the first group is the solvent and anaesthetic diethyl ether, commonly referred to simply as "ether". Ethers are common in organic chemistry and even more prevalent in biochemistry, as they are common linkages in carbohydrates and lignin.

Zinc chloride is the name of inorganic chemical compounds with the formula ZnCl2 and its hydrates. Zinc chlorides, of which nine crystalline forms are known, are colorless or white, and are highly soluble in water. This salt is hygroscopic and even deliquescent. Zinc chloride finds wide application in textile processing, metallurgical fluxes, and chemical synthesis. No mineral with this chemical composition is known aside from the very rare mineral simonkolleite, Zn5(OH)8Cl2·H2O.

Oxalyl chloride is an organic chemical compound with the formula (COCl)2. This colorless, sharp-smelling liquid, the diacyl chloride of oxalic acid, is a useful reagent in organic synthesis.
In organic chemistry, the Michael reaction or Michael addition describes a reaction between any Michael donor and any Michael acceptor. It belongs to the larger class of conjugate additions and is widely used for the mild formation of C–C bonds. Many asymmetric variants exist and depending on the conditions, Michael Additions can be diastereoselective and/or enantioselective.

The Simmons–Smith reaction is an organic cheletropic reaction involving an organozinc carbenoid that reacts with an alkene to form a cyclopropane. It is named after Howard Ensign Simmons, Jr. and Ronald D. Smith. It uses a methylene free radical intermediate that is delivered to both carbons of the alkene simultaneously, therefore the configuration of the double bond is preserved in the product and the reaction is stereospecific.
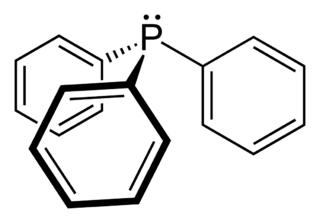
Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to PPh3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature. It dissolves in non-polar organic solvents such as benzene and diethyl ether.
Dimethylamine is an organic compound with the formula (CH3)2NH. This secondary amine is a colorless, flammable gas with an ammonia-like odor. Dimethylamine is commonly encountered commercially as a solution in water at concentrations up to around 40%. An estimated 270,000 tons were produced in 2005.

Sodium dithiophosphate is the salt with the formula Na3PS2O2. It is usually supplied as the hydrated solid or as an aqueous solution together with other thiophosphates such as sodium monothiophosphate and sodium trithiophosphate. It is a colorless compound, but commercial samples can appear dark owing to the presence of impurities. It is used to facilitate the isolation of molybdenum from its ores.

The Knorr pyrrole synthesis is a widely used chemical reaction that synthesizes substituted pyrroles (3). The method involves the reaction of an α-amino-ketone (1) and a compound containing an electron-withdrawing group α to a carbonyl group (2).

Zinc dialkyldithiophosphates are a family of coordination compounds developed in the 1940s that feature zinc bound to the anion of a dialkyldithiophosphoric salt. These uncharged compounds are not salts. They are soluble in nonpolar solvents, and the longer-chain derivatives easily dissolve in mineral and synthetic oils used as lubricants. They come under CAS number 68649-42-3. In aftermarket oil additives, the percentage of ZDDP ranges approximately between 2 and 15%. Zinc dithiophosphates have many names, including ZDDP, ZnDTP, and ZDP.

Phosphorus pentasulfide is the inorganic compound with the formula P2S5 or dimer P4S10. This yellow solid is the one of two phosphorus sulfides of commercial value. Samples often appear greenish-gray due to impurities. It is soluble in carbon disulfide but reacts with many other solvents such as alcohols, DMSO, and DMF.

Zinc phosphide (Zn3P2) is an inorganic chemical compound. It is a grey solid, although commercial samples are often dark or even black. It is used as a rodenticide. Zn3P2 is a II-V semiconductor with a direct band gap of 1.5 eV and may have applications in photovoltaic cells. A second compound exists in the zinc-phosphorus system, zinc diphosphide (ZnP2).

Organothiophosphates or organophosphorothioates are a subclass of organophosphorus compounds. Many are used as pesticides, some have medical applications, and some are used as oil additives. They generally have the chemical formula (RO)3PS, [(RO)2P(S)O]−, R(RO)2PS, etc.
Thiophosphates are chemical compounds and anions with the general chemical formula PS
4−xO3−
x and related derivatives where organic groups are attached to one or more O or S. Thiophosphates feature tetrahedral phosphorus(V) centers.
Compounds of zinc are chemical compounds containing the element zinc which is a member of the group 12 of the periodic table. The oxidation state of zinc in most compounds is the group oxidation state of +2. Zinc may be classified as a post-transition main group element with zinc(II). Zinc compounds are noteworthy for their nondescript behavior, they are generally colorless, do not readily engage in redox reactions, and generally adopt symmetrical structures.

Ammonium diethyl dithiophosphate or more systematically ammonium O,O′-diethyl dithiophosphate, is the ammonium salt of diethyl dithiophosphoric acid. It is used as a source of the (C2H5O)2PS2− ligand in coordination chemistry and in analytical chemistry for determination of various ions. It can be obtained by the reaction of phosphorus pentasulfide with ethanol and ammonia. In crystal structure of this compound the ammonium cation is connected by four charge-assisted N—H···S hydrogen bonds to four tetrahedral diethyl dithiophosphate anions.

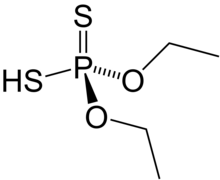
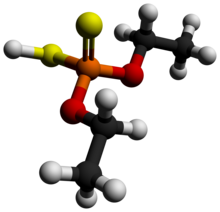
Dimethyl dithiophosphoric acid is the organophosphorus compound with the formula (CH3O)2PS2H. It is the processor for production of the organothiophosphate insecticide Malathion. Although samples can appear dark, the compound is a colorless, distillable liquid.

Diethylphosphite is the organophosphorus compound with the formula (C2H5O)2P(O)H. It is a popular reagent for generating other organophosphorus compounds, exploiting the high reactivity of the P-H bond. Diethylphosphite is a colorless liquid. The molecule is tetrahedral.

Diethyl oxomalonate is the diethyl ester of mesoxalic acid (ketomalonic acid), the simplest oxodicarboxylic acid and thus the first member (n = 0) of a homologous series HOOC–CO–(CH2)n–COOH with the higher homologues oxalacetic acid (n = 1), α-ketoglutaric acid (n = 2) and α-ketoadipic acid (n = 3) (the latter a metabolite of the amino acid lysine). Diethyl oxomalonate reacts because of its highly polarized keto group as electrophile in addition reactions and is a highly active reactant in pericyclic reactions such as the Diels-Alder reactions, cycloadditions or ene reactions. At humid air, mesoxalic acid diethyl ester reacts with water to give diethyl mesoxalate hydrate and the green-yellow oil are spontaneously converted to white crystals.
Zinc diphosphide (ZnP2) is an inorganic chemical compound. It is a red semiconductor solid with a band gap of 2.1 eV. It is one of the two compounds in the zinc-phosphorus system, the other being zinc phosphide (Zn3P2).