Ethers are a class of organic compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula R–O–R′, where R and R′ represent the alkyl or aryl groups. Ethers can again be classified into two varieties: if the alkyl or aryl groups are the same on both sides of the oxygen atom, then it is a simple or symmetrical ether, whereas if they are different, the ethers are called mixed or unsymmetrical ethers. A typical example of the first group is the solvent and anaesthetic diethyl ether, commonly referred to simply as "ether" (CH3–CH2–O–CH2–CH3). Ethers are common in organic chemistry and even more prevalent in biochemistry, as they are common linkages in carbohydrates and lignin.

An ester is a chemical compound derived from an oxoacid in which at least one –OH hydroxyl group is replaced by an –O– alkyl (alkoxy) group, as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils.
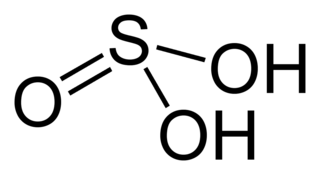
Sulfurous acid is the chemical compound with the formula H2SO3. There is no evidence that sulfurous acid exists in solution, but the molecule has been detected in the gas phase. The conjugate bases of this elusive acid are, however, common anions, bisulfite and sulfite. Sulfurous acid is an intermediate species in the formation of acid rain from sulfur dioxide.

Sulfites or sulphites are compounds that contain the sulfite ion, SO2−
3. The sulfite ion is the conjugate base of bisulfite. Although its acid is elusive, its salts are widely used.

Malonic acid (IUPAC systematic name: propanedioic acid) is a dicarboxylic acid with structure CH2(COOH)2. The ionized form of malonic acid, as well as its esters and salts, are known as malonates. For example, diethyl malonate is malonic acid's diethyl ester. The name originates from the Greek word μᾶλον (malon) meaning 'apple'.
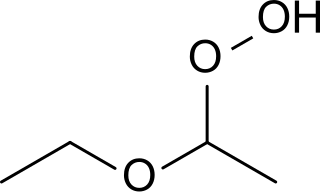
Diethyl ether hydroperoxide is the organic compound with the formula C2H5OCH(OOH)CH3. It is a colorless, distillable liquid. Diethyl ether hydroperoxide and its condensation products are blamed for the explosive organic peroxides that slowly form upon exposure of diethyl ether to ambient air and temperature conditions.

A sulfonic acid (or sulphonic acid) refers to a member of the class of organosulfur compounds with the general formula R−S(=O)2−OH, where R is an organic alkyl or aryl group and the S(=O)2(OH) group a sulfonyl hydroxide. As a substituent, it is known as a sulfo group. A sulfonic acid can be thought of as sulfuric acid with one hydroxyl group replaced by an organic substituent. The parent compound (with the organic substituent replaced by hydrogen) is the parent sulfonic acid, HS(=O)2(OH), a tautomer of sulfurous acid, S(=O)(OH)2. Salts or esters of sulfonic acids are called sulfonates.
In organic chemistry, the Michael reaction or Michael addition is the nucleophilic addition of a carbanion or another nucleophile to an α,β-unsaturated carbonyl compound containing an electron withdrawing group. It belongs to the larger class of conjugate additions and is widely used for the mild formation of C–C bonds. Many asymmetric variants exist.

Dimethyl sulfite is a sulfite ester with the chemical formula (CH3O)2SO.

A sulfoxide is a chemical compound containing a sulfinyl (SO) functional group attached to two carbon atoms. It is a polar functional group. Sulfoxides are an oxidized derivatives of sulfides. Examples of important sulfoxides are alliin, a precursor to the compound that gives freshly crushed garlic its aroma, and dimethyl sulfoxide (DMSO), a common solvent.

Caffeic acid is an organic compound that is classified as a hydroxycinnamic acid. This yellow solid consists of both phenolic and acrylic functional groups. It is found in all plants because it is an intermediate in the biosynthesis of lignin, one of the principal components of woody plant biomass and its residues.

The Knorr pyrrole synthesis is a widely used chemical reaction that synthesizes substituted pyrroles (3). The method involves the reaction of an α-amino-ketone (1) and a compound containing an electron-withdrawing group α to a carbonyl group (2).

The bisulfite ion (IUPAC-recommended nomenclature: hydrogensulfite) is the ion HSO−
3. Salts containing the HSO−
3 ion are also known as "sulfite lyes". Sodium bisulfite is used interchangeably with sodium metabisulfite (Na2S2O5). Sodium metabisulfite dissolves in water to give a solution of Na+HSO−
3.

Zinc dialkyldithiophosphates are a family of coordination compounds developed in the 1940s that feature zinc bound to the anion of a dialkyldithiophosphoric acid. These uncharged compounds are not salts. They are soluble in nonpolar solvents, and the longer-chain derivatives easily dissolve in mineral and synthetic oils used as lubricants. They come under CAS number 68649-42-3. In aftermarket oil additives, the percentage of ZDDP ranges approximately between 2 and 15%. Zinc dithiophosphates have many names, including ZDDP, ZnDTP, and ZDP.

Hydroxylammonium sulfate (NH3OH)2SO4, is the sulfuric acid salt of hydroxylamine. It is primarily used as an easily handled form of hydroxylamine, which is explosive when pure.
Polymer stabilizers are chemical additives which may be added to polymeric materials, such as plastics and rubbers, to inhibit or retard their degradation. Common polymer degradation processes include oxidation, UV-damage, thermal degradation, ozonolysis, combinations thereof such as photo-oxidation, as well as reactions with catalyst residues, dyes, or impurities. All of these degrade the polymer at a chemical level, via chain scission, uncontrolled recombination and cross-linking, which adversely affects many key properties such as strength, malleability, appearance and colour.

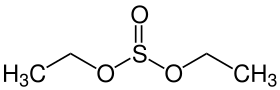
A sulfite ester is a functional group with the structure (RO)(R'O)SO. They adopt a trigonal pyramidal molecular geometry due to the presence of lone pairs on the sulphur atom.

Thiosulfurous acid (HS−S(=O)−OH) is a hypothetical compound with the formula S2(OH)2. Attempted synthesis leads to polymers. It is a low oxidation state (+1) sulfur acid. It is the equivalent acid for disulfur monoxide. Salts derived from thiosulfurous acid, which are also unknown, are named "thiosulfites" or "sulfurothioites". The ion is S=SO2−
2.

Wine preservatives are used to preserve the quality and shelf life of bottled wine without affecting its taste. Specifically, they are used to prevent oxidation and bacterial spoilage by inhibiting microbial activity.