Related Research Articles

Differential scanning calorimetry (DSC) is a thermoanalytical technique in which the difference in the amount of heat required to increase the temperature of a sample and reference is measured as a function of temperature. Both the sample and reference are maintained at nearly the same temperature throughout the experiment. Generally, the temperature program for a DSC analysis is designed such that the sample holder temperature increases linearly as a function of time. The reference sample should have a well-defined heat capacity over the range of temperatures to be scanned. Additionally, the reference sample must be stable, of high purity, and must not experience much change across the temperature scan. Typically, reference standards have been metals such as indium, tin, bismuth, and lead, but other standards such as polyethylene and fatty acids have been proposed to study polymers and organic compounds, respectively.
Thermal analysis is a branch of materials science where the properties of materials are studied as they change with temperature. Several methods are commonly used – these are distinguished from one another by the property which is measured:

Thermogravimetric analysis or thermal gravimetric analysis (TGA) is a method of thermal analysis in which the mass of a sample is measured over time as the temperature changes. This measurement provides information about physical phenomena, such as phase transitions, absorption, adsorption and desorption; as well as chemical phenomena including chemisorptions, thermal decomposition, and solid-gas reactions.

Ytterbium(III) oxide is the chemical compound with the formula Yb2O3. It is one of the more commonly encountered compounds of ytterbium. It occurs naturally in trace amounts in the mineral gadolinite. It was first isolated from this in 1878 by Jean Charles Galissard de Marignac.

Nickel(II) sulfate, or just nickel sulfate, usually refers to the inorganic compound with the formula NiSO4(H2O)6. This highly soluble blue green coloured salt is a common source of the Ni2+ ion for electroplating.

Ammonium tetrathiomolybdate is the chemical compound with the formula (NH4)2MoS4. This bright red ammonium salt is an important reagent in the chemistry of molybdenum and has been used as a building block in bioinorganic chemistry. The thiometallate (see metallate) anion has the distinctive property of undergoing oxidation at the sulfur centers concomitant with reduction of the metal from Mo(VI) to Mo(IV).

Ferrous oxalate (iron(II) oxalate) are inorganic compound with the formula FeC2O4(H2O)x where x is 0 or 2. These are orange compounds, poorly soluble in water.

Huntite is a carbonate mineral with the chemical formula Mg3Ca(CO3)4. Huntite crystallizes in the trigonal system and typically occurs as platy crystals and powdery masses. For most of recorded history its main use was as a white pigment. Today the most common industrial use of huntite is as a natural mixture with hydromagnesite as a flame retardant or fire retardant additive for polymers.
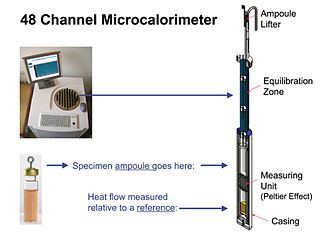
Isothermal microcalorimetry (IMC) is a laboratory method for real-time monitoring and dynamic analysis of chemical, physical and biological processes. Over a period of hours or days, IMC determines the onset, rate, extent and energetics of such processes for specimens in small ampoules at a constant set temperature.
Uranyl metaphosphate is a compound of uranium, phosphorus, and oxygen. It is one of the phosphates of uranium with the formula [UO2(PO3)2]n. This long-chain compound is formed via the thermal decomposition of UO2(H2PO4)2·3H2O. Double salts such as NaUO2(PO3)3 and CsUO2(PO3)3 are known.
Methylpyridinium is an ion with the formula C5H5NCH+3. It is the N-methylated derivative of pyridine. It confers no color to its salts. The ion is classified as an quaternary ammonium ion.
Chromium(II) oxalate is an inorganic compound with the chemical formula CrC2O4.

Bismuth oxynitrate is the name applied to a number of compounds that contain Bi3+, nitrate ions and oxide ions and which can be considered as compounds formed from Bi2O3, N2O5 and H2O. Other names for bismuth oxynitrate include bismuth subnitrate and bismuthyl nitrate. In older texts bismuth oxynitrate is often simply described as BiONO3 or basic bismuth nitrate. Bismuth oxynitrate was once called magisterium bismuti or bismutum subnitricum, and was used as a white pigment, in beauty care, and as a gentle disinfectant for internal and external use. It is also used to form Dragendorff's reagent, which is used as a TLC stain.

Bismuth(III) nitrate is a salt composed of bismuth in its cationic +3 oxidation state and nitrate anions. The most common solid form is the pentahydrate. It is used in the synthesis of other bismuth compounds. It is available commercially. It is the only nitrate salt formed by a group 15 element, indicative of bismuth's metallic nature.
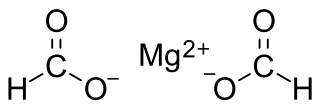
Magnesium formate is a magnesium salt of formic acid. It is an inorganic compound. It consists of a magnesium cation and formate anion. It can be prepared by reacting magnesium oxide with formic acid. The dihydrate is formed when crystallizing from the solution. The dihydrate dehydrates at 105 °C to form anhydrate, then decomposes at 500 °C to produce magnesium oxide. Magnesium formate can be used for organic syntheses.
The oxalatonickelates are a class of compounds that contain nickel complexed by oxalate groups. They form a series of double salts, and include clusters with multiple nickel atoms. Since oxalate functions as a bidentate ligand it can satisfy two coordinate positions around the nickel atom, or it can bridge two nickel atoms together.

Neodymium nitrate is an inorganic compound with the formula Nd(NO3)3·(x(H2O). It is typically encountered as the hexahydrate, Nd(NO3)3·6H2O, which is more accurately formulated as [Nd(NO3)3(H2O)4].2H2O to reflect the crystal structure. It decomposes to NdONO3 at elevated temperature.

Neodymium bismuthide or Bismuth-Neodymium is a binary inorganic compound of neodymium and bismuth with the formula NdBi. It forms crystals.

Europium(III) phosphate is one of the phosphates of europium, with the chemical formula of EuPO4. Other phosphates include europium(II) phosphate (Eu3(PO4)2) and europium(II,III) phosphate (Eu3Eu(PO4)3).
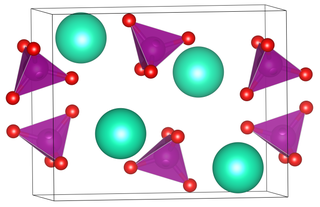
Caesium permanganate is the permanganate salt of caesium, with the chemical formula CsMnO4.
References
- ↑ Bhadeshia, H.K.D.H. "Thermal analyses techniques. Differential thermal analysis" (PDF). University of Cambridge, Material Science and Metallurgy. Retrieved 2023-09-17.
- ↑ Robert Bud, Deborah Jean Warner (1998). Instruments of Science. Taylor & Francis. pp. 170–171. ISBN 9780815315612.
- ↑ Ferrer, S.; Borras, J.; Martin-Gil, J.; Martin-Gil, F.J. (1989). "Thermal studies on sulphonamide derivative complexes". Thermochimica Acta. 147 (2). Elsevier BV: 321–330. doi:10.1016/0040-6031(89)85187-1. ISSN 0040-6031.
- ↑ Ferrer, S.; Borras, J.; Martin-Gil, J.; Martin-Gil, F.J. (1989). "Thermal decomposition of sulphonamide derivative complexes". Thermochimica Acta. 153. Elsevier BV: 205–220. doi:10.1016/0040-6031(89)85434-6. ISSN 0040-6031.
- ↑ Alzuet, G.; Ferrer, S.; Borrás, J.; Martín-Gil, J.; Martín-Gil, F.J. (1991). "Thermal studies of sulphonamide derivative complexes". Thermochimica Acta. 185 (2). Elsevier BV: 315–333. doi:10.1016/0040-6031(91)80053-l. ISSN 0040-6031.
- ↑ BERGER, K. G.; AKEHURST, E. E. (2007-06-28). "Some applications of differential thermal analysis to oils and fats". International Journal of Food Science & Technology. 1 (3). Wiley: 237–247. doi:10.1111/j.1365-2621.1966.tb01810.x. ISSN 0950-5423.
- ↑ C. Ramos-Sanchez, M; Rey, F.J.; L. Rodriguez, M; Martin-Gil, F.J.; Martin-Gil, J. (1988). "DTG and dta studies on typical sugars". Thermochimica Acta. 134. Elsevier BV: 55–60. doi:10.1016/0040-6031(88)85216-x. ISSN 0040-6031.
- ↑ Rey, F.J.; Ramos-Sanchez, M.C.; Rodriguez-Mendez, M.L.; Martin-Gil, J.; Martin-Gil, F.J. (1988). "DTG and DTA studies on sugar derivatives". Thermochimica Acta. 134. Elsevier BV: 67–72. doi:10.1016/0040-6031(88)85218-3. ISSN 0040-6031.
- ↑ L. Rodriguez-Mendez, Ma; Rey, F.J.; Martin-Gil, J.; Martin-Gil, F.J. (1988). "DTG and DTA studies on amino acids". Thermochimica Acta. 134. Elsevier BV: 73–78. doi:10.1016/0040-6031(88)85219-5. ISSN 0040-6031.
- ↑ Ramachandran V.S. “Applications of differential thermal analysis in cement chemistry”. Chap. V, Chemical Publishing Co., Inc., New York (1969), 92.
- ↑ Smykatz-Kloss, W. (1982). "Application of differential thermal analysis in mineralogy". Journal of Thermal Analysis. 23 (1–2). Springer Science and Business Media LLC: 15–44. doi:10.1007/bf01908484. ISSN 0022-5215. S2CID 93921297.
- ↑ Smykatz-Kloss, W.; Heil, A.; Kaeding, L.; Roller, E. (1991). "Thermal analysis in environmental studies". Thermal Analysis in the Geosciences. Lecture Notes in Earth Sciences. Vol. 38. Berlin/Heidelberg: Springer-Verlag. pp. 352–367. doi:10.1007/bfb0010275. ISBN 3-540-54520-4.
- ↑ Villanueva, PrE; Girela, F.; Castellanos, M. (1976). "The application of differential thermal analysis and thermogravimetric analysis to dating bone remains". Journal of Forensic Sciences. 21 (4): 822–830. doi:10.1520/JFS10567J. PMID 184235.
- ↑ Misiego-Tejeda J.C., Marcos-Contreras G.J., Sarabia Herrero F.J., Martín Gil J. and Martín Gil F.J. "Un horno doméstico de la primera Edad del Hierro de "El Soto de Medinilla" (Valladolid) y su análisis por ATD". BSAA (University of Valladolid) 1993, LIX, 89 111.
- ↑ KINGERY, W. D. (1974). "A note on the differential thermal analysis of archaeological ceramics". Archaeometry. 16 (1). Wiley: 109–112. doi:10.1111/j.1475-4754.1974.tb01099.x. ISSN 0003-813X.