The phosphonium cation describes polyatomic cations with the chemical formula PR+
4. These cations have tetrahedral structures. The salts are generally colorless or take the color of the anions.

Phosphorus pentachloride is the chemical compound with the formula PCl5. It is one of the most important phosphorus chlorides, others being PCl3 and POCl3. PCl5 finds use as a chlorinating reagent. It is a colourless, water-sensitive and moisture-sensitive solid, although commercial samples can be yellowish and contaminated with hydrogen chloride.

Phosphorus trichloride is a inorganic compound with the chemical formula PCl3. A colorless liquid when pure, it is an important industrial chemical, being used for the manufacture of phosphites and other organophosphorus compounds. It is toxic and reacts readily with water to release hydrogen chloride.
Phosphorus trifluoride (formula PF3), is a colorless and odorless gas. It is highly toxic and reacts slowly with water. Its main use is as a ligand in metal complexes. As a ligand, it parallels carbon monoxide in metal carbonyls, and indeed its toxicity is due to its binding with the iron in blood hemoglobin in a similar way to carbon monoxide.

Phosphorus triiodide (PI3) is an inorganic compound with the formula PI3. A red solid, it is a common misconception that PI3 is too unstable to be stored; it is, in fact, commercially available. It is widely used in organic chemistry for converting alcohols to alkyl iodides. It is also a powerful reducing agent. Note that phosphorus also forms a lower iodide, P2I4, but the existence of PI5 is doubtful at room temperature.
Organophosphorus compounds are organic compounds containing phosphorus. They are used primarily in pest control as an alternative to chlorinated hydrocarbons that persist in the environment. Some organophosphorus compounds are highly effective insecticides, although some are extremely toxic to humans, including sarin and VX nerve agents.

Arsenic trichloride is an inorganic compound with the formula AsCl3, also known as arsenous chloride or butter of arsenic. This poisonous oil is colourless, although impure samples may appear yellow. It is an intermediate in the manufacture of organoarsenic compounds.
Organophosphines are organophosphorus compounds with the formula PRnH3−n, where R is an organic substituent. These compounds can be classified according to the value of n: primary phosphines (n = 1), secondary phosphines (n = 2), tertiary phosphines (n = 3). All adopt pyramidal structures. Organophosphines are generally colorless, lipophilic liquids or solids. The parent of the organophosphines is phosphine (PH3).

Thiophosphoryl chloride is an inorganic compound with the formula PSCl3. It is a colorless pungent smelling liquid that fumes in air. It is synthesized from phosphorus chloride and used to thiophosphorylate organic compounds, such as to produce insecticides.

Triphenyl phosphite is the organophosphorus compound with the formula P(OC6H5)3. It is a colourless viscous liquid.
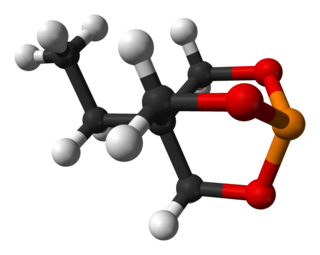
Trimethylolpropane phosphite, C2H5C(CH2O)3P, is a phosphite ester used as a ligand in organometallic chemistry. Trimethylolpropane phosphite is sometimes abbreviated to EtCage. It is a white solid that is soluble in organic solvents. It is also highly toxic.

Triethyl phosphite is an organophosphorus compound with the formula P(OCH2CH3)3, often abbreviated P(OEt)3. It is a colorless, malodorous liquid. It is used as a ligand in organometallic chemistry and as a reagent in organic synthesis
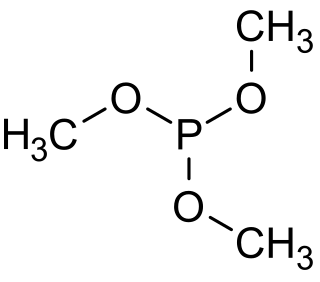
Trimethyl phosphite is an organophosphorus compound with the formula P(OCH3)3, often abbreviated P(OMe)3. It is a colorless liquid with a highly pungent odor. It is used as a ligand in organometallic chemistry and as a reagent in organic synthesis. The molecule features a pyramidal phosphorus(III) center bound to three methoxy groups.

Chlorodiphenylphosphine is an organophosphorus compound with the formula (C6H5)2PCl, abbreviated Ph2PCl. It is a colourless oily liquid with a pungent odor that is often described as being garlic-like and detectable even in the ppb range. It is useful reagent for introducing the Ph2P group into molecules, which includes many ligands. Like other halophosphines, Ph2PCl is reactive with many nucleophiles such as water and easily oxidized even by air.

Dichlorophenylphosphine is an organophosphorus compound with the formula C6H5PCl2. This colourless viscous liquid is commonly used in the synthesis of organophosphines.

Chlorodiisopropylphosphine is an organophosphorus compound with the formula [(CH3)2CH]2PCl. It is a colorless liquid that reacts with water and oxygen. The compound is used to prepare tertiary phosphines and phosphinite ligands.

Diethylphosphite is the organophosphorus compound with the formula (C2H5O)2P(O)H. It is a popular reagent for generating other organophosphorus compounds, exploiting the high reactivity of the P-H bond. Diethylphosphite is a colorless liquid. The molecule is tetrahedral.
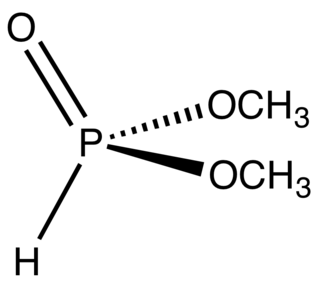
Dimethyl hydrogen phosphite (DMHP), also known as Dimethylphosphite, is an organophosphorus compound with the formula (CH3O)2P(O)H. It is a reagent for generating other organophosphorus compounds, exploiting the high reactivity of the P-H bond. The molecule is tetrahedral. It is a colorless liquid. The compounds can be prepared by methanolysis of phosphorus trichloride or by heating diethylphosphite in methanol.

Methyldichlorophosphine (alternatively known as dichloro(methyl)phosphane and methyl phosphonous dichloride) is an organophosphorus compound with the chemical formula CH3PCl2. It is a colorless, corrosive, flammable, and highly reactive liquid with a pungent odor.
In organophosphorus chemistry, the Kinnear–Perren reaction (sometimes the Clay-Kinnear-Perren reaction) is used to prepare alkylphosphonyl dichlorides (RP(O)Cl2) and alkylphosphonate esters (RP(O)(OR')2). The reactants are alkyl chloride, phosphorus trichloride, and aluminium trichloride as catalyst. The reaction proceeds via the alkyltrichlorophosphonium salt:















