
Organic electronics is a field of materials science concerning the design, synthesis, characterization, and application of organic molecules or polymers that show desirable electronic properties such as conductivity. Unlike conventional inorganic conductors and semiconductors, organic electronic materials are constructed from organic (carbon-based) molecules or polymers using synthetic strategies developed in the context of organic chemistry and polymer chemistry.
Molecular electronics is the study and application of molecular building blocks for the fabrication of electronic components. It is an interdisciplinary area that spans physics, chemistry, and materials science. The unifying feature is use of molecular building blocks to fabricate electronic components. Due to the prospect of size reduction in electronics offered by molecular-level control of properties, molecular electronics has generated much excitement. It provides a potential means to extend Moore's Law beyond the foreseen limits of small-scale conventional silicon integrated circuits.
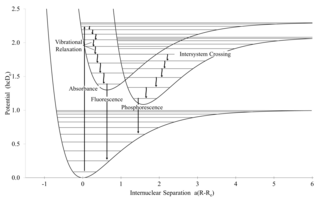
Intersystem crossing (ISC) is an isoenergetic radiationless process involving a transition between the two electronic states with different spin multiplicity.
Organic semiconductors are solids whose building blocks are pi-bonded molecules or polymers made up by carbon and hydrogen atoms and – at times – heteroatoms such as nitrogen, sulfur and oxygen. They exist in the form of molecular crystals or amorphous thin films. In general, they are electrical insulators, but become semiconducting when charges are either injected from appropriate electrodes, upon doping or by photoexcitation.

Pentacene is a polycyclic aromatic hydrocarbon consisting of five linearly-fused benzene rings. This highly conjugated compound is an organic semiconductor. The compound generates excitons upon absorption of ultra-violet (UV) or visible light; this makes it very sensitive to oxidation. For this reason, this compound, which is a purple powder, slowly degrades upon exposure to air and light.
Hybrid solar cells combine advantages of both organic and inorganic semiconductors. Hybrid photovoltaics have organic materials that consist of conjugated polymers that absorb light as the donor and transport holes. Inorganic materials in hybrid cells are used as the acceptor and electron transporter in the structure. The hybrid photovoltaic devices have a potential for not only low-cost by roll-to-roll processing but also for scalable solar power conversion.
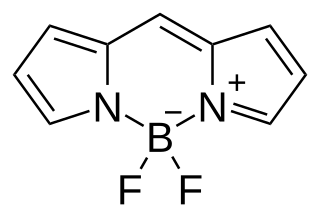
BODIPY is the technical common name of a chemical compound with formula C
9H
7BN
2F
2, whose molecule consists of a boron difluoride group BF
2 joined to a dipyrromethene group C
9H
7N
2; specifically, the compound 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene in the IUPAC nomenclature. The common name is an abbreviation for "boron-dipyrromethene". It is a red crystalline solid, stable at ambient temperature, soluble in methanol.
Organic photovoltaic devices (OPVs) are fabricated from thin films of organic semiconductors, such as polymers and small-molecule compounds, and are typically on the order of 100 nm thick. Because polymer based OPVs can be made using a coating process such as spin coating or inkjet printing, they are an attractive option for inexpensively covering large areas as well as flexible plastic surfaces. A promising low cost alternative to conventional solar cells made of crystalline silicon, there is a large amount of research being dedicated throughout industry and academia towards developing OPVs and increasing their power conversion efficiency.
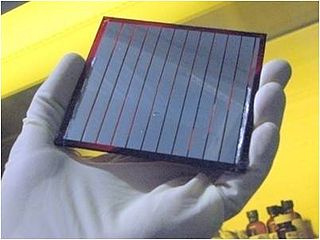
An organic solar cell (OSC) or plastic solar cell is a type of photovoltaic that uses organic electronics, a branch of electronics that deals with conductive organic polymers or small organic molecules, for light absorption and charge transport to produce electricity from sunlight by the photovoltaic effect. Most organic photovoltaic cells are polymer solar cells.

Polyfluorene is a polymer with formula (C13H8)n, consisting of fluorene units linked in a linear chain — specifically, at carbon atoms 2 and 7 in the standard fluorene numbering. It can also be described as a chain of benzene rings linked in para positions with an extra methylene bridge connecting every pair of rings.

Photoconductive atomic force microscopy (PC-AFM) is a variant of atomic force microscopy that measures photoconductivity in addition to surface forces.
Mesoporous organosilica are a type of silica containing organic groups that give rise to mesoporosity. They exhibit pore size ranging from 2 nm - 50 nm, depending on the organic substituents. In contrast, zeolites exhibit pore sizes less than a nanometer. PMOs have potential applications as catalysts, adsorbents, trapping agents, drug delivery agents, stationary phases in chromatography and chemical sensors.

Polymer-fullerene bulk heterojunction solar cells are a type of solar cell researched in academic laboratories. Polymer-fullerene solar cells are a subset of organic solar cells, also known as organic photovoltaic (OPV) cells, which use organic materials as their active component to convert solar radiation into electrical energy. The polymer, which functions as the donor material in these solar cells, and fullerene derivatives, which function as the acceptor material, are essential components. Specifically, fullerene derivatives act as electron acceptors for donor materials like P3HT, creating a polymer-fullerene based photovoltaic cell. The Polymer-fullerene BHJ forms two channels for transferring electrons and holes to the corresponding electrodes, as opposed to the planar architecture when the Acceptor (A) and Donor (D) materials were sequentially stacked on top of each other and could selectively touch the cathode and anode electrodes. Hence, the D and A domains are expected to form a bi-continuous network with Nano-scale morphology for efficient charge transport and collection after exciton dissociation. Therefore, in the BHJ device architecture, a mixture of D and A molecules in the same or different solvents was used to form a bi-continual layer, which serves as the active layer of the device that absorbs light for exciton generation. The bi-continuous three-dimensional interpenetrating network of the BHJ design generates a greater D-A interface, which is necessary for effective exciton dissociation in the BHJ due to short exciton diffusion. When compared to the prior bilayer design, photo-generated excitons may dissociate into free holes and electrons more effectively, resulting in better charge separation for improved performance of the cell.

In organic chemistry, contorted aromatics, or more precisely contorted polycyclic aromatic hydrocarbons, are polycyclic aromatic hydrocarbons (PAHs) in which the fused aromatic molecules deviate from the usual planarity.
Luis M. Campos is a Professor in the Department of Chemistry at Columbia University. Campos leads a research team focused on nanostructured materials, macromolecular systems, and single-molecule electronics.

Robert P. H. Chang is an American materials scientist who served as the president of the Materials Research Society (1989) and as a general secretary and president of the International Union of Materials Research Societies (IUMRS). Currently Chang heads the Materials Research Institute at Northwestern University. He is a member of advisory boards of the National Institute for Materials Science and of the journal Science and Technology of Advanced Materials.
Light harvesting materials harvest solar energy that can then be converted into chemical energy through photochemical processes. Synthetic light harvesting materials are inspired by photosynthetic biological systems such as light harvesting complexes and pigments that are present in plants and some photosynthetic bacteria. The dynamic and efficient antenna complexes that are present in photosynthetic organisms has inspired the design of synthetic light harvesting materials that mimic light harvesting machinery in biological systems. Examples of synthetic light harvesting materials are dendrimers, porphyrin arrays and assemblies, organic gels, biosynthetic and synthetic peptides, organic-inorganic hybrid materials, and semiconductor materials. Synthetic and biosynthetic light harvesting materials have applications in photovoltaics, photocatalysis, and photopolymerization.
Non-fullerene acceptors (NFAs) are types of acceptors used in organic solar cells (OSCs). The name Fullerene comes from another type of acceptor-molecule which was used as the main acceptor material for bulk heterojunction Organic solar cells. Non-fullerene acceptors are thus defined as not being a part of this sort of acceptors.

Kevin Sivula is a highly cited American chemical engineer and researcher in the field of solar cells. He is a professor of molecular engineering at EPFL and the head of the Laboratory for Molecular Engineering of Optoelectronic Nanomaterials at EPFL's School of Basic Sciences.

Fred Wudl is an American materials scientist, academic researcher. He is a Professor Emeritus in the Department of Materials Engineering at the University of California, Santa Barbara.












