
Black sigatoka is a leaf-spot disease of banana plants caused by the ascomycete fungus Mycosphaerella fijiensis (Morelet), also known as black leaf streak. It was discovered in 1963 and named for its similarities with yellow Sigatoka, which is caused by Mycosphaerella musicola (Mulder), which was itself named after the Sigatoka Valley in Fiji. In the same valley an outbreak of this disease reached epidemic proportions from 1912 to 1923.

Grape black rot is a fungal disease caused by an ascomycetous fungus, Guignardia bidwellii, that attacks grape vines during hot and humid weather. “Grape black rot originated in eastern North America, but now occurs in portions of Europe, South America, and Asia. It can cause complete crop loss in warm, humid climates, but is virtually unknown in regions with arid summers.” The name comes from the black fringe that borders growing brown patches on the leaves. The disease also attacks other parts of the plant, “all green parts of the vine: the shoots, leaf and fruit stems, tendrils, and fruit. The most damaging effect is to the fruit”.

Phomopsis cane and leaf spot occurs wherever grapes are grown. Phomopsis cane and leaf spot is more severe in grape-growing regions characterized by a humid temperate climate through the growing season. Crop losses up to 30% have been reported to be caused by Phomopsis cane and leaf spot.
Leptosphaeria coniothyrium is a plant pathogen. It can be found around the world.

Mycosphaerella brassicicola is a plant pathogen. The pathogen is the teleomorph phase of an ascomycete fungus, which causes the ring spot disease of brassicas. The supplementary anamorph phase Asteromella brassicae produces conidia through its asexual reproduction, however these spores are not confirmed to cause disease in host plants.
Ascochyta tarda or Phoma tarda is a fungal plant pathogen that causes dieback and leafspot on coffee and was first observed in Ethiopia in 1954. It poses a potentially serious threat to coffee crops, but climate change may reduce the prevalence of environmental conditions favorable to its spread.

Diplocarpon earlianum is a species of fungus that causes disease in strawberry plants called strawberry leaf scorch. The disease overwinters in plant debris and infects strawberry plants during the spring season when it is wet. The five main methods to reduce strawberry leaf scorch include: irrigation techniques, crop rotation, planting resistant and disease-free seeds, fungicide use, and sanitation measures. Control of strawberry leaf scorch is important because it is responsible for the majority of disease in strawberries. Diplocarpon earliana affects the fruit quality and yield of the strawberry crop. Losses range from negligible to severe depending on numerous epidemiological factors including cultivar susceptibility, type of cropping system, and weather conditions

Ascochyta is a genus of ascomycete fungi, containing several species that are pathogenic to plants, particularly cereal crops. The taxonomy of this genus is still incomplete. The genus was first described in 1830 by Marie-Anne Libert, who regarded the spores as minute asci and the cell contents as spherical spores. Numerous revisions to the members of the genus and its description were made for the next several years. Species that are plant pathogenic on cereals include, A. hordei, A. graminea, A. sorghi, A. tritici. Symptoms are usually elliptical spots that are initially chlorotic and later become a necrotic brown. Management includes fungicide applications and sanitation of diseased plant tissue debris.
Septoria cannabis is a species of plant pathogen from the genus Septoria that causes the disease commonly known as Septoria leaf spot. Early symptoms of infection are concentric white lesions on the vegetative leaves of cannabis plants, followed by chlorosis and necrosis of the leaf until it is ultimately overcome by disease and all living cells are then killed. Septoria, which is an ascomycete and pycnidia producing fungus, has been well known to attack Solanaceae and Cucurbitaceae species as well as many tree species. This genus is known to comprise over 1,000 species of pathogens, each infecting a specific and unique host.

Didymella bryoniae, syn. Mycosphaerella melonis, is an ascomycete fungal plant pathogen that causes gummy stem blight on the family Cucurbitaceae, which includes cantaloupe, cucumber, muskmelon and watermelon plants. The anamorph/asexual stage for this fungus is called Phoma cucurbitacearum. When this pathogen infects the fruit of cucurbits it is called black rot.

Elsinoë ampelina is a plant pathogen, which is the causal agent of anthracnose on grape.

Dibotryon morbosum or Apiosporina morbosa is a plant pathogen, which is the causal agent of black knot. It affects members of the Prunus genus such as; cherry, plum, apricot, and chokecherry trees in North America. The disease produces rough, black growths that encircle and kill the infested parts, and provide habitat for insects.

Botrytis fabae is a plant pathogen, a fungus that causes chocolate spot disease of broad or fava bean plants, Vicia faba. It was described scientifically by Mexican-born Galician microbiologist Juan Rodríguez Sardiña in 1929.

Monilinia oxycocci (Woronin) Honey,, common names cranberry cottonball, cranberry hard rot, tip blight, is a fungal infection of large cranberry and small cranberry. The tips of young flowering shoots wilt before they flower. Fruit that forms on the plant can then be infected by the asexual spores traveling through the plant, causing the berries to harden, turn cottony on the inside, and dry out instead of maturing. The berries are filled with a cotton-like fungus and are generally yellowish with tan stripes or blotches at maturity, making them unmarketable. It results in important economic impacts on many cranberry marshes, particularly in Wisconsin.
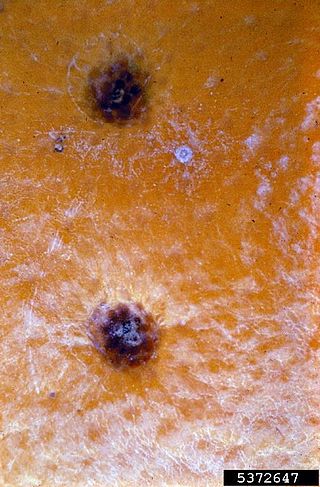
Citrus black spot is a fungal disease caused by Phyllosticta citricarpa(previously known as Guignardia citricarpa). This Ascomycete fungus affects citrus plants throughout subtropical climates, causing a reduction in both fruit quantity and quality.
Raspberry leaf spot is a plant disease caused by Sphaerulina rubi, an ascomycete fungus. Early symptoms of infection are dark green spots on young leaves. As the disease progresses, these spots turn tan or gray in color. Disease management strategies for raspberry leaf spots include the use of genetically resistant raspberry plant varieties, chemical fungicide sprays, and cultural practices such as pruning and thinning out canes.

Cherry leaf spot is a fungal disease which infects cherries and plums. Sweet, sour, and ornamental cherries are susceptible to the disease, being most prevalent in sour cherries. The variety of sour cherries that is the most susceptible are the English morello cherries. This is considered a serious disease in the Midwest, New England states, and Canada. It has also been estimated to infect 80 percent of orchards in the Eastern states. It must be controlled yearly to avoid a significant loss of the crop. If not controlled properly, the disease can dramatically reduce yields by nearly 100 percent. The disease is also known as yellow leaf or shothole disease to cherry growers due to the characteristic yellowing leaves and shot holes present in the leaves upon severe infection.
Gummy stem blight is a cucurbit-rot disease caused by the fungal plant pathogen Didymella bryoniae. Gummy stem blight can affect a host at any stage of growth in its development and affects all parts of the host including leaves, stems and fruits. Symptoms generally consist of circular dark tan lesions that blight the leaf, water soaked leaves, stem cankers, and gummy brown ooze that exudes from cankers, giving it the name gummy stem blight. Gummy stem blight reduces yields of edible cucurbits by devastating the vines and leaves and rotting the fruits. There are various methods to control gummy stem blight, including use of treated seed, crop rotation, using preventative fungicides, eradication of diseased material, and deep plowing previous debris.

Pecan scab is the most economically significant disease of pecan trees in the southeastern United States. Venturia effusa is a fungal plant pathogen that causes pecan scab. The fungus causes lesions and tissue death on pecan twigs, petioles, leaves, nuts and shucks beginning in early spring, with multiple cycles of infection repeating until late summer. Wind and rain spread the fungus to a susceptible host. Control of the disease is achieved by fungicide, sanitation and, in some cases, quarantine.
Phyllosticta minima is a fungus of the division Ascomycota which causes purple-bordered leaf spot, a largely cosmetic disease that infects maple trees. It grows on living and fallen leaves, creating tan, ovular lesions 1⁄4 inch in diameter and ringed with 'purple' or black spores.














