
In chemistry, alcohol is an organic compound that carries at least one hydroxyl functional group (−OH) bound to a saturated carbon atom. The term alcohol originally referred to the primary alcohol ethanol (ethyl alcohol), which is used as a drug and is the main alcohol present in alcoholic drinks. An important class of alcohols, of which methanol and ethanol are the simplest members, includes all compounds for which the general formula is CnH2n+1OH. Simple monoalcohols that are the subject of this article include primary (RCH2OH), secondary (R2CHOH) and tertiary (R3COH) alcohols.

In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic.

In chemistry, an aldehyde is an organic compound containing a functional group with the structure −C(H)=O. The functional group itself is known as an aldehyde or formyl group. Aldehydes are common and play important roles in the technology and biological spheres.
In chemistry, a hydration reaction is a chemical reaction in which a substance combines with water. In organic chemistry, water is added to an unsaturated substrate, which is usually an alkene or an alkyne. This type of reaction is employed industrially to produce ethanol, isopropanol, and butan-2-ol.
1-Hexanol (IUPAC name hexan-1-ol) is an organic alcohol with a six-carbon chain and a condensed structural formula of CH3(CH2)5OH. This colorless liquid is slightly soluble in water, but miscible with diethyl ether and ethanol. Two additional straight chain isomers of 1-hexanol, 2-hexanol and 3-hexanol, exist, both of which differing by the location of the hydroxyl group. Many isomeric alcohols have the formula C6H13OH. It is used in the perfume industry.
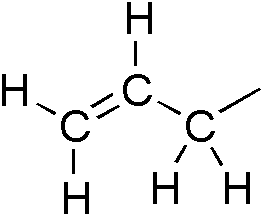
An allyl group is a substituent with the structural formula H2C=CH−CH2R, where R is the rest of the molecule. It consists of a methylene bridge (−CH2−) attached to a vinyl group (−CH=CH2). The name is derived from the Latin word for garlic, Allium sativum. In 1844, Theodor Wertheim isolated an allyl derivative from garlic oil and named it "Schwefelallyl". The term allyl applies to many compounds related to H2C=CH−CH2, some of which are of practical or of everyday importance, for example, allyl chloride.
Hydroformylation, also known as oxo synthesis or oxo process, is an industrial process for the production of aldehydes from alkenes. This chemical reaction entails the net addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention: Production capacity reached 6.6×106 tons in 1995. It is important because aldehydes are easily converted into many secondary products. For example, the resulting aldehydes are hydrogenated to alcohols that are converted to detergents. Hydroformylation is also used in speciality chemicals, relevant to the organic synthesis of fragrances and drugs. The development of hydroformylation is one of the premier achievements of 20th-century industrial chemistry.
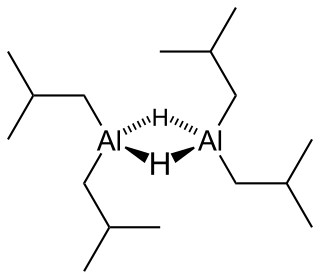
Diisobutylaluminium hydride (DIBALH, DIBAL, DIBAL-H or DIBAH) is a reducing agent with the formula (i-Bu2AlH)2, where i-Bu represents isobutyl (-CH2CH(CH3)2). This organoaluminium compound was investigated originally as a co-catalyst for the polymerization of alkenes.

Zinc cyanide is the inorganic compound with the formula Zn(CN)2. It is a white solid that is used mainly for electroplating zinc but also has more specialized applications for the synthesis of organic compounds.
Undecylenic acid is an organic compound with the formula CH2=CH(CH2)8CO2H. It is an unsaturated fatty acid. It is a colorless oil. Undecylenic acid is mainly used for the production of Nylon-11 and in the treatment of fungal infections of the skin, but it is also a precursor in the manufacture of many pharmaceuticals, personal hygiene products, cosmetics, and perfumes. Salts and esters of undecylenic acid are known as undecylenates.

Tributylphosphine is the organophosphorus compound with the formula P(C
4H
9)
3. Abbreviated or PBu
3, it is a tertiary phosphine. It is an oily liquid at room temperature, with a nauseating odor. It reacts slowly with atmospheric oxygen, and rapidly with other oxidizing agents, to give the corresponding phosphine oxide. It is usually handled using air-free techniques.
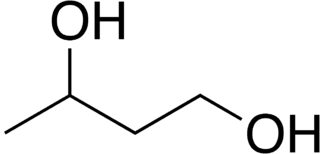
1,3-Butanediol is an organic compound with the formula CH3CH(OH)CH2CH2OH. With two alcohol functional groups, the molecule is classified as a diol. The compound is a colorless, water-soluble liquid. It is one of four common structural isomers of butanediol. It has no large scale uses.
Octanal is the organic compound, an aldehyde, with the chemical formula CH3(CH2)6CHO. A colorless fragrant liquid with a fruit-like odor, it occurs naturally in citrus oils. It is used commercially as a component in perfumes and in flavor production for the food industry. It is usually produced by hydroformylation of heptene and the dehydrogenation of 1-octanol.
Nonanal, also called nonanaldehyde, pelargonaldehyde or Aldehyde C-9, is an aldehyde. A colourless, oily liquid, nonanal is a component of perfumes. Although it occurs in several natural oils, it is produced commercially by hydroformylation of 1-octene.

Phenylacetaldehyde is an organic compound used in the synthesis of fragrances and polymers. Phenylacetaldehyde is an aldehyde that consists of acetaldehyde bearing a phenyl substituent; the parent member of the phenylacetaldehyde class of compounds. It has a role as a human metabolite, a Saccharomyces cerevisiae metabolite, an Escherichia coli metabolite and a mouse metabolite. It is an alpha-CH2-containing aldehyde and a member of phenylacetaldehydes.

Succinaldehyde or succindialdehyde is an organic compound with the formula (CH2CHO)2. Typical of other dialdehydes, succinaldehyde is highly reactive and is rarely observed as the dialdehyde. Usually, it is handled as the hydrates or methanol-derived acetal. It is a precursor to tropinone. Succinaldehyde can used as a crosslinking agent for proteins, but it is less widely used than the related dialdehyde glutaraldehyde.
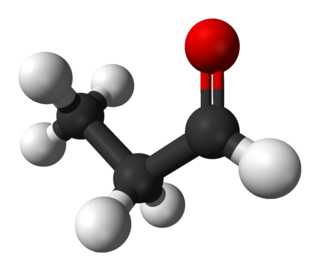
Propionaldehyde or propanal is the organic compound with the formula CH3CH2CHO. It is the 3-carbon aldehyde. It is a colourless, flammable liquid with a slightly fruity odour. It is produced on a large scale industrially.
Fatty aldehydes are aliphatic, long-chain aldehydes which may be mono- or polyunsaturated. The fatty aldehydes include compounds such as octanal, nonanal, decanal or dodecanal. The nomenclature is derived from the nomenclature of the alkanes, the ending -al is added to indicate the aldehyde group.
Alkynylation is an addition reaction in organic synthesis where a terminal alkyne adds to a carbonyl group to form an α-alkynyl alcohol. When the acetylide is formed from acetylene, the reaction gives an α-ethynyl alcohol. This process is often referred to as ethynylation. Such process often involve metal acetylide intermediates
Hydroxymethylation is a chemical reaction that installs the CH2OH group. The transformation can be implemented in many ways and applies to both industrial and biochemical processes.












