Related Research Articles

In multicellular organisms, stem cells are undifferentiated or partially differentiated cells that can change into various types of cells and proliferate indefinitely to produce more of the same stem cell. They are the earliest type of cell in a cell lineage. They are found in both embryonic and adult organisms, but they have slightly different properties in each. They are usually distinguished from progenitor cells, which cannot divide indefinitely, and precursor or blast cells, which are usually committed to differentiating into one cell type.
Transdifferentiation, also known as lineage reprogramming, is the process in which one mature somatic cell is transformed into another mature somatic cell without undergoing an intermediate pluripotent state or progenitor cell type. It is a type of metaplasia, which includes all cell fate switches, including the interconversion of stem cells. Current uses of transdifferentiation include disease modeling and drug discovery and in the future may include gene therapy and regenerative medicine. The term 'transdifferentiation' was originally coined by Selman and Kafatos in 1974 to describe a change in cell properties as cuticle producing cells became salt-secreting cells in silk moths undergoing metamorphosis.
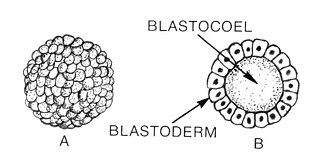
Blastulation is the stage in early animal embryonic development that produces the blastula. In mammalian development the blastula develops into the blastocyst with a differentiated inner cell mass and an outer trophectoderm. The blastula is a hollow sphere of cells known as blastomeres surrounding an inner fluid-filled cavity called the blastocoel. Embryonic development begins with a sperm fertilizing an egg cell to become a zygote, which undergoes many cleavages to develop into a ball of cells called a morula. Only when the blastocoel is formed does the early embryo become a blastula. The blastula precedes the formation of the gastrula in which the germ layers of the embryo form.

Embryonic stem cells (ESCs) are pluripotent stem cells derived from the inner cell mass of a blastocyst, an early-stage pre-implantation embryo. Human embryos reach the blastocyst stage 4–5 days post fertilization, at which time they consist of 50–150 cells. Isolating the inner cell mass (embryoblast) using immunosurgery results in destruction of the blastocyst, a process which raises ethical issues, including whether or not embryos at the pre-implantation stage have the same moral considerations as embryos in the post-implantation stage of development.

Oct-4, also known as POU5F1, is a protein that in humans is encoded by the POU5F1 gene. Oct-4 is a homeodomain transcription factor of the POU family. It is critically involved in the self-renewal of undifferentiated embryonic stem cells. As such, it is frequently used as a marker for undifferentiated cells. Oct-4 expression must be closely regulated; too much or too little will cause differentiation of the cells.

An organoid is a miniaturised and simplified version of an organ produced in vitro in three dimensions that mimics the key functional, structural and biological complexity of that organ. They are derived from one or a few cells from a tissue, embryonic stem cells or induced pluripotent stem cells, which can self-organize in three-dimensional culture owing to their self-renewal and differentiation capacities. The technique for growing organoids has rapidly improved since the early 2010s, and The Scientist names it as one of the biggest scientific advancements of 2013. Scientists and engineers use organoids to study development and disease in the laboratory, drug discovery and development in industry, personalized diagnostics and medicine, gene and cell therapies, tissue engineering and regenerative medicine.

Induced pluripotent stem cells are a type of pluripotent stem cell that can be generated directly from a somatic cell. The iPSC technology was pioneered by Shinya Yamanaka and Kazutoshi Takahashi in Kyoto, Japan, who together showed in 2006 that the introduction of four specific genes, collectively known as Yamanaka factors, encoding transcription factors could convert somatic cells into pluripotent stem cells. Shinya Yamanaka was awarded the 2012 Nobel Prize along with Sir John Gurdon "for the discovery that mature cells can be reprogrammed to become pluripotent."

PDX1, also known as insulin promoter factor 1, is a transcription factor in the ParaHox gene cluster. In vertebrates, Pdx1 is necessary for pancreatic development, including β-cell maturation, and duodenal differentiation. In humans this protein is encoded by the PDX1 gene, which was formerly known as IPF1. The gene was originally identified in the clawed frog Xenopus laevis and is present widely across the evolutionary diversity of bilaterian animals, although it has been lost in evolution in arthropods and nematodes. Despite the gene name being Pdx1, there is no Pdx2 gene in most animals; single-copy Pdx1 orthologs have been identified in all mammals. Coelacanth and cartilaginous fish are, so far, the only vertebrates shown to have two Pdx genes, Pdx1 and Pdx2.
Neurogenins, often abbreviated as Ngn, are a family of bHLH transcription factors involved in specifying neuronal differentiation. The family consisting of Neurogenin-1, Neurogenin-2, and Neurogenin-3, plays a fundamental role in specifying neural precursor cells and regulating the differentiation of neurons during embryonic development. It is one of many gene families related to the atonal gene in Drosophila. Other positive regulators of neuronal differentiation also expressed during early neural development include NeuroD and ASCL1.

Neurogenin-3 (NGN3) is a protein that in humans is encoded by the Neurog3 gene.

Cell potency is a cell's ability to differentiate into other cell types. The more cell types a cell can differentiate into, the greater its potency. Potency is also described as the gene activation potential within a cell, which like a continuum, begins with totipotency to designate a cell with the most differentiation potential, pluripotency, multipotency, oligopotency, and finally unipotency.
Induced stem cells (iSC) are stem cells derived from somatic, reproductive, pluripotent or other cell types by deliberate epigenetic reprogramming. They are classified as either totipotent (iTC), pluripotent (iPSC) or progenitor or unipotent – (iUSC) according to their developmental potential and degree of dedifferentiation. Progenitors are obtained by so-called direct reprogramming or directed differentiation and are also called induced somatic stem cells.
Directed differentiation is a bioengineering methodology at the interface of stem cell biology, developmental biology and tissue engineering. It is essentially harnessing the potential of stem cells by constraining their differentiation in vitro toward a specific cell type or tissue of interest. Stem cells are by definition pluripotent, able to differentiate into several cell types such as neurons, cardiomyocytes, hepatocytes, etc. Efficient directed differentiation requires a detailed understanding of the lineage and cell fate decision, often provided by developmental biology.

Chromatin assembly factor-1 (CAF-1) is a protein complex — including Chaf1a (p150), Chaf1b (p60), and p48 subunits in humans, or Cac1, Cac2, and Cac3, respectively, in yeast— that assembles histone tetramers onto replicating DNA during the S phase of the cell cycle.

Pancreatic progenitor cells are multipotent stem cells originating from the developing fore-gut endoderm which have the ability to differentiate into the lineage specific progenitors responsible for the developing pancreas.

Richard Paul Harvey is a molecular biologist, the Sir Peter Finley professor of Heart Research at the University of New South Wales and Deputy Director and Head of the Developmental and Stem Cell Biology Division at the Victor Chang Cardiac Research Institute.

Bradley E. Bernstein is a biologist and Professor of Cell Biology at Harvard Medical School. He is Chair of the Department of Cancer Biology at the Dana–Farber Cancer Institute and the Director of the Broad Institute's Gene Regulation Observatory. He is known for contributions to the fields of epigenetics and cancer biology.

Derrick J. Rossi, is a Canadian stem cell biologist and entrepreneur. He is a co-founder of the pharmaceutical company Moderna.

Yi Zhang is a Chinese-American biochemist who specializes in the fields of epigenetics, chromatin, and developmental reprogramming. He is a Fred Rosen Professor of Pediatrics and professor of genetics at Harvard Medical School, a senior investigator of Program in Cellular and Molecular Medicine at Boston Children's Hospital, and an investigator of the Howard Hughes Medical Institute. He is also an associate member of the Harvard Stem Cell Institute, as well as the Broad Institute of MIT and Harvard. He is best known for his discovery of several classes of epigenetic enzymes and the identification of epigenetic barriers of SCNT cloning.

The dorsal lip of the blastopore is a structure that forms during early embryonic development and is important for its role in organizing the germ layers. The dorsal lip is formed during early gastrulation as folding of tissue along the involuting marginal zone of the blastocoel forms an opening known as the blastopore. It is particularly important for its role in neural induction through the default model, where signaling from the dorsal lip protects a region of the epiblast from becoming epidermis, thus allowing it to develop to its default neural tissue.
References
- 1 2 J B Gurdon; D A Melton (1981). "Gene transfer in amphibian eggs and oocytes". Annual Review of Genetics . 15 (1): 189–218. doi:10.1146/ANNUREV.GE.15.120181.001201. ISSN 0066-4197. PMID 7039494. Wikidata Q28277421.
- ↑ R P Harvey; D A Melton (3 June 1988). "Microinjection of synthetic Xhox-1A homeobox mRNA disrupts somite formation in developing Xenopus embryos". Cell . 53 (5): 687–97. doi:10.1016/0092-8674(88)90087-6. ISSN 0092-8674. PMID 2897242. Wikidata Q28283555.
- ↑ M R Rebagliati; Daniel L Weeks; Richard P. Harvey; D A Melton (1 October 1985). "Identification and cloning of localized maternal RNAs from Xenopus eggs". Cell . 42 (3): 769–777. doi:10.1016/0092-8674(85)90273-9. ISSN 0092-8674. PMID 2414011. Wikidata Q28300491.
- ↑ Kaspar Mossman (26 May 2009). "Profile of Clifford Tabin". Proceedings of the National Academy of Sciences of the United States of America . 106 (21): 8407–9. Bibcode:2009PNAS..106.8407M. doi: 10.1073/PNAS.0903946106 . ISSN 0027-8424. PMC 2688980 . PMID 19458049. Wikidata Q28245644.
- ↑ https://www.hhmi.org/scientists/douglas-melton
- ↑ Douglas A. Melton, Harvard University
- ↑ Fox, Michael J. (May 3, 2007). "The 2007 Time 100: Douglas Melton". Time .
- ↑ FitzPatrick, Lauren (June 3, 2007). "Time Will Tell - Scientist from Blue Island honored by Time Magazine". SouthtownStar . 37: a3.
- ↑ Doug Melton's Curriculum Vitae
- ↑ Melton, Douglas (1979). The expression of transfer RNA genes to other DNAs microinjected into Xenopus oocytes (PhD thesis). University of Cambridge.
- ↑ Hemmati-Brivanlou A; Melton D (1 January 1997). "Vertebrate embryonic cells will become nerve cells unless told otherwise". Cell . 88 (1): 13–17. doi: 10.1016/S0092-8674(00)81853-X . ISSN 0092-8674. PMID 9019398. Wikidata Q34415315.
- ↑ D A Melton; P. A. Krieg; M. R. Rebagliati; T. Maniatis; K. Zinn; M. R. Green (25 September 1984). "Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter". Nucleic Acids Research . 12 (18): 7035–56. doi:10.1093/NAR/12.18.7035. ISSN 0305-1048. PMC 320141 . PMID 6091052. Wikidata Q27861016.
- ↑ Chad A. Cowan; Irina Klimanskaya; Jill McMahon; et al. (25 March 2004). "Derivation of Embryonic Stem-Cell Lines from Human Blastocysts". The New England Journal of Medicine . 350 (13): 1353–1356. doi:10.1056/NEJMSR040330. ISSN 0028-4793. PMID 14999088. S2CID 10575047. Wikidata Q29999326.
- ↑ Derivation of Human Embryonic Stem Cells by Immunosurgery
- ↑ Qiao Zhou; Juliana Brown; Andrew Kanarek; Jayaraj Rajagopal; Douglas A Melton (2 October 2008). "In vivo reprogramming of adult pancreatic exocrine cells to beta-cells". Nature . 455 (7213): 627–32. Bibcode:2008Natur.455..627Z. doi:10.1038/NATURE07314. ISSN 1476-4687. PMID 18754011. Wikidata Q28292190.
- ↑ Felicia W Pagliuca; Jeffrey R Millman; Mads Gürtler; et al. (1 October 2014). "Generation of functional human pancreatic β cells in vitro". Cell . 159 (2): 428–439. doi:10.1016/J.CELL.2014.09.040. ISSN 0092-8674. PMC 4617632 . PMID 25303535. Wikidata Q28249536.
- ↑ "Douglas Melton, noted stem cell researcher, leaves Harvard for Vertex to create diabetes treatments". STAT. 2022-04-05. Retrieved 2022-04-05.
- ↑ Michael J Fox (1 May 2007). "Time 100 scientists & thinkers. Douglas Melton". Time . 169 (20): 121. ISSN 0040-781X. PMID 17536327. Wikidata Q28304304.
- ↑ PhD, Dana G. Smith (2016-09-27). "2016 Ogawa-Yamanaka Stem Cell Prize Awarded to Douglas Melton". Gladstone Institutes. Retrieved 2017-06-29.
- ↑ Prize, Abarca. "PROF. DOUGLAS A. MELTON, WINNER OF THE III EDITION OF THE 'ABARCA PRIZE' FOR HIS DISRUPTIVE CURE OF TYPE 1 DIABETES, IS RECEIVED BY HIS MAJESTY THE KING OF SPAIN". www.prnewswire.com. Retrieved 2024-02-13.