Related Research Articles
Abiotic stress is the negative impact of non-living factors on the living organisms in a specific environment. The non-living variable must influence the environment beyond its normal range of variation to adversely affect the population performance or individual physiology of the organism in a significant way.

In botany, a stoma, also called a stomate, is a pore found in the epidermis of leaves, stems, and other organs, that controls the rate of gas exchange between the internal air spaces of the leaf and the atmosphere. The pore is bordered by a pair of specialized parenchyma cells known as guard cells that regulate the size of the stomatal opening.

Plant hormones are signal molecules, produced within plants, that occur in extremely low concentrations. Plant hormones control all aspects of plant growth and development, including embryogenesis, the regulation of organ size, pathogen defense, stress tolerance and reproductive development. Unlike in animals each plant cell is capable of producing hormones. Went and Thimann coined the term "phytohormone" and used it in the title of their 1937 book.

C4 carbon fixation or the Hatch–Slack pathway is one of three known photosynthetic processes of carbon fixation in plants. It owes the names to the 1960s discovery by Marshall Davidson Hatch and Charles Roger Slack.

Photorespiration (also known as the oxidative photosynthetic carbon cycle or C2 cycle) refers to a process in plant metabolism where the enzyme RuBisCO oxygenates RuBP, wasting some of the energy produced by photosynthesis. The desired reaction is the addition of carbon dioxide to RuBP (carboxylation), a key step in the Calvin–Benson cycle, but approximately 25% of reactions by RuBisCO instead add oxygen to RuBP (oxygenation), creating a product that cannot be used within the Calvin–Benson cycle. This process lowers the efficiency of photosynthesis, potentially lowering photosynthetic output by 25% in C3 plants. Photorespiration involves a complex network of enzyme reactions that exchange metabolites between chloroplasts, leaf peroxisomes and mitochondria.
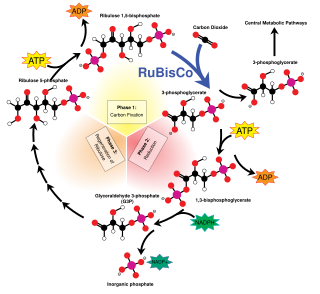
C3 carbon fixation is the most common of three metabolic pathways for carbon fixation in photosynthesis, the other two being C4 and CAM. This process converts carbon dioxide and ribulose bisphosphate (RuBP, a 5-carbon sugar) into two molecules of 3-phosphoglycerate through the following reaction:

Abscisic acid is a plant hormone. ABA functions in many plant developmental processes, including seed and bud dormancy, the control of organ size and stomatal closure. It is especially important for plants in the response to environmental stresses, including drought, soil salinity, cold tolerance, freezing tolerance, heat stress and heavy metal ion tolerance.
Moisture stress is a form of abiotic stress that occurs when the moisture of plant tissues is reduced to suboptimal levels. Water stress occurs in response to atmospheric and soil water availability when the transpiration rate exceeds the rate of water uptake by the roots and cells lose turgor pressure. Moisture stress is described by two main metrics, water potential and water content.

Guard cells are specialized plant cells in the epidermis of leaves, stems and other organs that are used to control gas exchange. They are produced in pairs with a gap between them that forms a stomatal pore. The stomatal pores are largest when water is freely available and the guard cells become turgid, and closed when water availability is critically low and the guard cells become flaccid. Photosynthesis depends on the diffusion of carbon dioxide (CO2) from the air through the stomata into the mesophyll tissues. Oxygen (O2), produced as a byproduct of photosynthesis, exits the plant via the stomata. When the stomata are open, water is lost by evaporation and must be replaced via the transpiration stream, with water taken up by the roots. Plants must balance the amount of CO2 absorbed from the air with the water loss through the stomatal pores, and this is achieved by both active and passive control of guard cell turgor pressure and stomatal pore size.
In botany, drought tolerance is the ability by which a plant maintains its biomass production during arid or drought conditions. Some plants are naturally adapted to dry conditions, surviving with protection mechanisms such as desiccation tolerance, detoxification, or repair of xylem embolism. Other plants, specifically crops like corn, wheat, and rice, have become increasingly tolerant to drought with new varieties created via genetic engineering. From an evolutionary perspective, the type of mycorrhizal associations formed in the roots of plants can determine how fast plants can adapt to drought.
A xerophyte is a species of plant that has adaptations to survive in an environment with little liquid water. Examples of xerophytes include cacti, pineapple and some gymnosperm plants. The morphology and physiology of xerophytes are adapted to conserve water during dry periods. Some species called resurrection plants can survive long periods of extreme dryness or desiccation of their tissues, during which their metabolic activity may effectively shut down. Plants with such morphological and physiological adaptations are said to be xeromorphic. Xerophytes such as cacti are capable of withstanding extended periods of dry conditions as they have deep-spreading roots and capacity to store water. Their waxy, thorny leaves prevent loss of moisture.

Transpiration is the process of water movement through a plant and its evaporation from aerial parts, such as leaves, stems and flowers. It is a passive process that requires no energy expense by the plant. Transpiration also cools plants, changes osmotic pressure of cells, and enables mass flow of mineral nutrients. When water uptake by the roots is less than the water lost to the atmosphere by evaporation plants close small pores called stomata to decrease water loss, which slows down nutrient uptake and decreases CO2 absorption from the atmosphere limiting metabolic processes, photosynthesis, and growth.
Dehydrin (DHN) is a multi-family of proteins present in plants that is produced in response to cold and drought stress. DHNs are hydrophilic, reliably thermostable, and disordered. They are stress proteins with a high number of charged amino acids that belong to the Group II Late Embryogenesis Abundant (LEA) family. DHNs are primarily found in the cytoplasm and nucleus but more recently, they have been found in other organelles, like mitochondria and chloroplasts.

Photosynthesis systems are electronic scientific instruments designed for non-destructive measurement of photosynthetic rates in the field. Photosynthesis systems are commonly used in agronomic and environmental research, as well as studies of the global carbon cycle.
Water-use efficiency (WUE) refers to the ratio of plant biomass to water lost by transpiration, can be defined either at the leaf, at the whole plant or a population/stand/field level:
Stomatal conductance, usually measured in mmol m−2 s−1 by a porometer, estimates the rate of gas exchange and transpiration through the leaf stomata as determined by the degree of stomatal aperture.
Breeding for drought resistance is the process of breeding plants with the goal of reducing the impact of dehydration on plant growth.
Hydraulic signals in plants are detected as changes in the organism's water potential that are caused by environmental stress like drought or wounding. The cohesion and tension properties of water allow for these water potential changes to be transmitted throughout the plant.

Alarm photosynthesis is a variation of photosynthesis where calcium oxalate crystals function as dynamic carbon pools, supplying carbon dioxide (CO2) to photosynthetic cells when stomata are partially or totally closed. This biochemical appendance of the photosynthetic machinery is a means to alleviate the perpetual plant dilemma of using atmospheric CO2 for photosynthesis and losing water vapor, or saving water and reducing photosynthesis. The function of alarm photosynthesis seems to be rather auxiliary to the overall photosynthetic performance. It supports a low photosynthetic rate, aiming at the maintenance and photoprotection of the photosynthetic apparatus rather than a substantial carbon gain.

Cannabis (/ˈkænəbɪs/) is commonly known as marijuana or hemp and has two known strains: Cannabis sativa and Cannabis indica, both of which produce chemicals to deter herbivory. The chemical composition includes specialized terpenes and cannabinoids, mainly tetrahydrocannabinol (THC), and cannabidiol (CBD). These substances play a role in defending the plant from pathogens including insects, fungi, viruses and bacteria. THC and CBD are stored mostly in the trichomes of the plant, and can cause psychological and physical impairment in the user, via the endocannabinoid system and unique receptors. THC increases dopamine levels in the brain, which attributes to the euphoric and relaxed feelings cannabis provides. As THC is a secondary metabolite, it poses no known effects towards plant development, growth, and reproduction. However, some studies show secondary metabolites such as cannabinoids, flavonoids, and terpenes are used as defense mechanisms against biotic and abiotic environmental stressors.
References
- ↑ Goyal, A.; Ahmed, M. (November 2012). "Barley: Production, Improvement, and Uses". Crop Science. 52 (6): 2852–2854. doi:10.2135/cropsci2012.12.0003bra. ISSN 0011-183X. S2CID 252135699.
- ↑ Varshney, Rajeev (2013). Translational Genomics for Crop Breeding : Volume 2 - Improvement for Abiotic Stress, Quality and Yield Improvement. Wiley-Blackwell. ISBN 978-1-299-87149-6. OCLC 858653470.
- ↑ Verstegen, Harold; Köneke, Otto; Korzun, Viktor; von Broock, Reinhard (2014), Kumlehn, Jochen; Stein, Nils (eds.), "The World Importance of Barley and Challenges to Further Improvements", Biotechnological Approaches to Barley Improvement, vol. 69, Berlin, Heidelberg: Springer Berlin Heidelberg, pp. 3–19, doi:10.1007/978-3-662-44406-1_1, ISBN 978-3-662-44405-4 , retrieved 2022-12-05
- ↑ Swanston, J. Stuart (2011-07-29). "Barley: Production, Improvement and Uses. Edited by S. E. Ullrich, Chichester, UK: Wiley-Blackwell (2011), pp. 637, £170.00. ISBN 978-0-8138-0123-0". Experimental Agriculture. 47 (4): 733. doi:10.1017/s0014479711000615. ISSN 0014-4797. S2CID 83513924.
- ↑ Wiegmann, Mathias; Maurer, Andreas; Pham, Anh; March, Timothy J.; Al-Abdallat, Ayed; Thomas, William T. B.; Bull, Hazel J.; Shahid, Mohammed; Eglinton, Jason; Baum, Michael; Flavell, Andrew J.; Tester, Mark; Pillen, Klaus (December 2019). "Barley yield formation under abiotic stress depends on the interplay between flowering time genes and environmental cues". Scientific Reports. 9 (1): 6397. Bibcode:2019NatSR...9.6397W. doi:10.1038/s41598-019-42673-1. ISSN 2045-2322. PMC 6484077 . PMID 31024028.
- ↑ Walker, A.; et al. (2021). Integrating the evidence for a terrestrial carbon sink caused by increasing atmospheric CO2. Umeå universitet, Institutionen för medicinsk kemi och biofysik. OCLC 1234762992.
- ↑ Finkelstein, Ruth (2013-11-01). "Abscisic Acid Synthesis and Response". The Arabidopsis Book. 11: e0166. doi:10.1199/tab.0166. ISSN 1543-8120. PMC 3833200 . PMID 24273463.
- ↑ Muhammad Aslam, Mehtab; Waseem, Muhammad; Jakada, Bello Hassan; Okal, Eyalira Jacob; Lei, Zuliang; Saqib, Hafiz Sohaib Ahmad; Yuan, Wei; Xu, Weifeng; Zhang, Qian (2022-01-19). "Mechanisms of Abscisic Acid-Mediated Drought Stress Responses in Plants". International Journal of Molecular Sciences. 23 (3): 1084. doi: 10.3390/ijms23031084 . ISSN 1422-0067. PMC 8835272 . PMID 35163008.
- ↑ Popova, L. P.; Lazova, G. N. (1990), "Carbonic Anhydrase Activity in Barley Leaves After Treatment with Abscisic Acid and Jasmonic Acid", Current Research in Photosynthesis, Dordrecht: Springer Netherlands, pp. 3273–3277, doi:10.1007/978-94-009-0511-5_737, ISBN 978-94-010-6716-4 , retrieved 2022-12-05
- ↑ Chloupek, O.; Dostál, V.; Středa, T.; Psota, V.; Dvořáčková, O. (December 2010). "Drought tolerance of barley varieties in relation to their root system size: Drought tolerance and roots size of barley". Plant Breeding. 129 (6): 630–636. doi:10.1111/j.1439-0523.2010.01801.x.
- ↑ Matamoros, M. A.; Loscos, J.; Dietz, K.-J.; Aparicio-Tejo, P. M.; Becana, M. (2010-01-01). "Function of antioxidant enzymes and metabolites during maturation of pea fruits". Journal of Experimental Botany. 61 (1): 87–97. doi:10.1093/jxb/erp285. ISSN 0022-0957. PMC 2791115 . PMID 19822534.
- 1 2 Hughes, Jon; Hepworth, Christopher; Dutton, Chris; Dunn, Jessica A.; Hunt, Lee; Stephens, Jennifer; Waugh, Robbie; Cameron, Duncan D.; Gray, Julie E. (June 2017). "Reducing Stomatal Density in Barley Improves Drought Tolerance without Impacting on Yield". Plant Physiology. 174 (2): 776–787. doi:10.1104/pp.16.01844. ISSN 0032-0889. PMC 5462017 . PMID 28461401.
- 1 2 Montilla-Bascón, Gracia; Rubiales, Diego; Hebelstrup, Kim H.; Mandon, Julien; Harren, Frans J. M.; Cristescu, Simona M.; Mur, Luis A. J.; Prats, Elena (2017-10-17). "Reduced nitric oxide levels during drought stress promote drought tolerance in barley and is associated with elevated polyamine biosynthesis". Scientific Reports. 7 (1): 13311. Bibcode:2017NatSR...713311M. doi:10.1038/s41598-017-13458-1. ISSN 2045-2322. PMC 5645388 . PMID 29042616. S2CID 205612254.
- ↑ Cai, Kangfeng; Chen, Xiaohui; Han, Zhigang; Wu, Xiaojian; Zhang, Shuo; Li, Qi; Nazir, Muhammad Mudassir; Zhang, Guoping; Zeng, Fanrong (2020). "Screening of Worldwide Barley Collection for Drought Tolerance: The Assessment of Various Physiological Measures as the Selection Criteria". Frontiers in Plant Science. 11: 1159. doi: 10.3389/fpls.2020.01159 . ISSN 1664-462X. PMC 7403471 . PMID 32849716.
- ↑ Thabet, Samar G.; Moursi, Yasser S.; Karam, Mohamed A.; Graner, Andreas; Alqudah, Ahmad M. (2018-11-02). "Genetic basis of drought tolerance during seed germination in barley". PLOS ONE. 13 (11): e0206682. Bibcode:2018PLoSO..1306682T. doi: 10.1371/journal.pone.0206682 . ISSN 1932-6203. PMC 6214555 . PMID 30388157.
- ↑ Wendelboe-Nelson, Charlotte; Morris, Peter C. (November 2012). "Proteins linked to drought tolerance revealed by DIGE analysis of drought resistant and susceptible barley varieties". Proteomics. 12 (22): 3374–3385. doi:10.1002/pmic.201200154. PMID 23001927. S2CID 29301162.
- ↑ Shakhatreh, Y.; Kafawin, O.; Ceccarelli, S.; Saoub, H. (2001-04-22). "Selection of Barley Lines for Drought Tolerance in Low-Rainfall Areas". Journal of Agronomy and Crop Science. 186 (2): 119–127. Bibcode:2001JAgCS.186..119S. doi:10.1046/j.1439-037x.2001.00459.x. ISSN 0931-2250.
- ↑ Fu, Daolin; Huang, Bingru; Xiao, Yanmei; Muthukrishnan, Subbaratnam; Liang, George H. (2007-04-01). "Overexpression of barley hva1 gene in creeping bentgrass for improving drought tolerance". Plant Cell Reports. 26 (4): 467–477. doi:10.1007/s00299-006-0258-7. ISSN 1432-203X. PMID 17106681. S2CID 494394.
- ↑ Rohila, Jai S; Jain, Rajinder K; Wu, Ray (2002-09-01). "Genetic improvement of Basmati rice for salt and drought tolerance by regulated expression of a barley Hva1 cDNA". Plant Science. 163 (3): 525–532. doi:10.1016/S0168-9452(02)00155-3. ISSN 0168-9452.
- ↑ Alexander, Ross D.; Wendelboe-Nelson, Charlotte; Morris, Peter C. (2019-09-01). "The barley transcription factor HvMYB1 is a positive regulator of drought tolerance". Plant Physiology and Biochemistry. 142: 246–253. doi:10.1016/j.plaphy.2019.07.014. ISSN 0981-9428. PMID 31374377. S2CID 199387889.
- ↑ Feng, Xue; Liu, Wenxing; Qiu, Cheng-Wei; Zeng, Fanrong; Wang, Yizhou; Zhang, Guoping; Chen, Zhong-Hua; Wu, Feibo (August 2020). "HvAKT2 and HvHAK1 confer drought tolerance in barley through enhanced leaf mesophyll H + homoeostasis". Plant Biotechnology Journal. 18 (8): 1683–1696. doi:10.1111/pbi.13332. ISSN 1467-7644. PMC 7336388 . PMID 31917885.