Related Research Articles

Coeliac disease or celiac disease is a long-term autoimmune disorder, primarily affecting the small intestine, where individuals develop intolerance to gluten, present in foods such as wheat, rye and barley. Classic symptoms include gastrointestinal problems such as chronic diarrhoea, abdominal distention, malabsorption, loss of appetite, and among children failure to grow normally. This often begins between six months and two years of age. Non-classic symptoms are more common, especially in people older than two years. There may be mild or absent gastrointestinal symptoms, a wide number of symptoms involving any part of the body, or no obvious symptoms. Coeliac disease was first described in childhood; however, it may develop at any age. It is associated with other autoimmune diseases, such as Type 1 diabetes mellitus and Hashimoto's thyroiditis, among others.
Peptic ulcer disease is a break in the inner lining of the stomach, the first part of the small intestine, or sometimes the lower esophagus. An ulcer in the stomach is called a gastric ulcer, while one in the first part of the intestines is a duodenal ulcer. The most common symptoms of a duodenal ulcer are waking at night with upper abdominal pain, and upper abdominal pain that improves with eating. With a gastric ulcer, the pain may worsen with eating. The pain is often described as a burning or dull ache. Other symptoms include belching, vomiting, weight loss, or poor appetite. About a third of older people have no symptoms. Complications may include bleeding, perforation, and blockage of the stomach. Bleeding occurs in as many as 15% of cases.
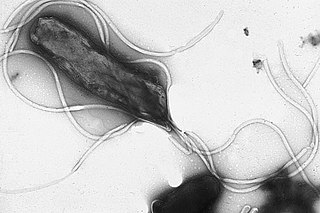
Helicobacter pylori, previously known as Campylobacter pylori, is a gram-negative, flagellated, helical bacterium. Mutants can have a rod or curved rod shape, and are less effective. Its helical body is thought to have evolved in order to penetrate the mucous lining of the stomach, helped by its flagella, and thereby establish infection. The bacterium was first identified as the causal agent of gastric ulcers in 1983 by the Australian doctors Barry Marshall and Robin Warren.
Indigestion, also known as dyspepsia or upset stomach, is a condition of impaired digestion. Symptoms may include upper abdominal fullness, heartburn, nausea, belching, or upper abdominal pain. People may also experience feeling full earlier than expected when eating. Indigestion is relatively common, affecting 20% of people at some point during their life, and is frequently caused by gastroesophageal reflux disease (GERD) or gastritis.
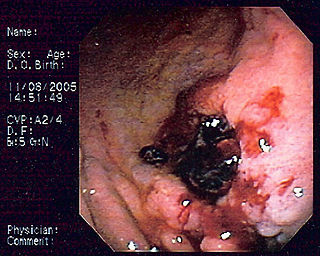
MALT lymphoma is a form of lymphoma involving the mucosa-associated lymphoid tissue (MALT), frequently of the stomach, but virtually any mucosal site can be affected. It is a cancer originating from B cells in the marginal zone of the MALT, and is also called extranodal marginal zone B cell lymphoma.
HLA-DQ (DQ) is a cell surface receptor protein found on antigen-presenting cells. It is an αβ heterodimer of type MHC class II. The α and β chains are encoded by two loci, HLA-DQA1 and HLA-DQB1, that are adjacent to each other on chromosome band 6p21.3. Both α-chain and β-chain vary greatly. A person often produces two α-chain and two β-chain variants and thus 4 isoforms of DQ. The DQ loci are in close genetic linkage to HLA-DR, and less closely linked to HLA-DP, HLA-A, HLA-B and HLA-C.
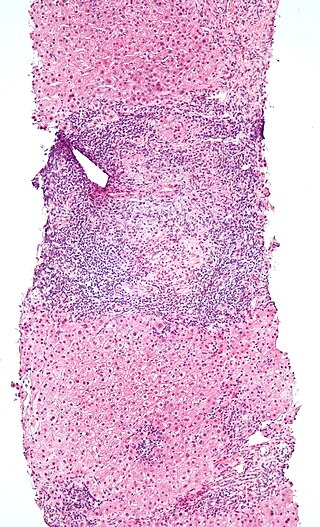
Intraepithelial lymphocytes (IEL) are lymphocytes found in the epithelial layer of mammalian mucosal linings, such as the gastrointestinal (GI) tract and reproductive tract. However, unlike other T cells, IELs do not need priming. Upon encountering antigens, they immediately release cytokines and cause killing of infected target cells. In the GI tract, they are components of gut-associated lymphoid tissue (GALT).

Duodenitis is inflammation of the duodenum. It may persist acutely or chronically.

Gluten-related disorders is the term for the diseases triggered by gluten, including celiac disease (CD), non-celiac gluten sensitivity (NCGS), gluten ataxia, dermatitis herpetiformis (DH) and wheat allergy. The umbrella category has also been referred to as gluten intolerance, though a multi-disciplinary physician-led study, based in part on the 2011 International Coeliac Disease Symposium, concluded that the use of this term should be avoided due to a lack of specificity.
Gluten-sensitive enteropathy–associated conditions are comorbidities or complications of gluten-related gastrointestinal distress. GSE has key symptoms typically restricted to the bowel and associated tissues; however, there are a wide variety of associated conditions. These include bowel disorders, eosinophilic gastroenteritis and increase with coeliac disease (CD) severity. With some early onset and a large percentage of late onset disease, other disorders appear prior to the coeliac diagnosis or allergic-like responses markedly increased in GSE. Many of these disorders persist on a strict gluten-free diet, and are thus independent of coeliac disease after triggering. For example, autoimmune thyroiditis is a common finding with GSE.
Anti-transglutaminase antibodies (ATA) are autoantibodies against the transglutaminase protein. Antibodies serve an important role in the immune system by detecting cells and substances that the rest of the immune system then eliminates. These cells and substances can be foreign and also can be produced by the body. Antibodies against the body's own products are called autoantibodies. Autoantibodies can sometimes errantly be directed against healthy portions of the organism, causing autoimmune diseases.
HLA-DQ2 (DQ2) is a serotype group within HLA-DQ (DQ) serotyping system. The serotype is determined by the antibody recognition of β2 subset of DQ β-chains. The β-chain of DQ is encoded by HLA-DQB1 locus and DQ2 are encoded by the HLA-DQB1*02 allele group. This group currently contains two common alleles, DQB1*0201 and DQB1*0202. HLA-DQ2 and HLA-DQB1*02 are almost synonymous in meaning. DQ2 β-chains combine with α-chains, encoded by genetically linked HLA-DQA1 alleles, to form the cis-haplotype isoforms. These isoforms, nicknamed DQ2.2 and DQ2.5, are also encoded by the DQA1*0201 and DQA1*0501 genes, respectively.

Enteropathy-associated T-cell lymphoma (EATL), previously termed enteropathy-associated T-cell lymphoma, type I and at one time termed enteropathy-type T-cell lymphoma (ETTL), is a complication of coeliac disease in which a malignant T-cell lymphoma develops in areas of the small intestine affected by the disease's intense inflammation. While a relatively rare disease, it is the most common type of primary gastrointestinal T-cell lymphoma.
Oat sensitivity represents a sensitivity to the proteins found in oats, Avena sativa. Sensitivity to oats can manifest as a result of allergy to oat seed storage proteins either inhaled or ingested. A more complex condition affects individuals who have gluten-sensitive enteropathy in which there is an autoimmune response to avenin, the glutinous protein in oats similar to the gluten within wheat. Sensitivity to oat foods can also result from their frequent contamination by wheat, barley, or rye particles.
The immunochemistry of Triticeae glutens is important in several inflammatory diseases. It can be subdivided into innate responses, class II mediated presentation, class I mediated stimulation of killer cells, and antibody recognition. The responses to gluten proteins and polypeptide regions differs according to the type of gluten sensitivity. The response is also dependent on the genetic makeup of the human leukocyte antigen genes. In gluten sensitive enteropathy, there are four types of recognition, innate immunity, HLA-DQ, and antibody recognition of gliadin and transglutaminase. With idiopathic gluten sensitivity only antibody recognition to gliadin has been resolved. In wheat allergy, the response pathways are mediated through IgE against other wheat proteins and other forms of gliadin.

Dermatitis herpetiformis (DH) is a chronic autoimmune blistering skin condition, characterised by intensely itchy blisters filled with a watery fluid. DH is a cutaneous manifestation of coeliac disease, although the exact causal mechanism is not known. DH is neither related to nor caused by herpes virus; the name means that it is a skin inflammation having an appearance similar to herpes.
HLA A1-B8-DR3-DQ2 haplotype is a multigene haplotype that covers a majority of the human major histocompatibility complex on chromosome 6. A multigene haplotype is set of inherited alleles covering several genes, or gene-alleles; common multigene haplotypes are generally the result of descent by common ancestry. Chromosomal recombination fragments multigene haplotypes as the distance to that ancestor increases in number of generations.
Non-celiac gluten sensitivity (NCGS) or gluten sensitivity is a controversial disorder which can cause both gastrointestinal and other problems.
The gluten challenge test is a medical test in which gluten-containing foods are consumed and (re-)occurrence of symptoms is observed afterwards to determine whether and how much a person reacts to these foods. The test may be performed in people with suspected gluten-related disorders in very specific occasions and under medical supervision, for example in people who had started a gluten-free diet without performing duodenal biopsy.
Ludvig M. Sollid is a Norwegian physician-scientist whose laboratory has made discoveries in the pathogenesis of HLA associated human disorders, most notably celiac disease. He is currently a Professor of Medicine (immunology) at the University of Oslo and a Senior Consultant at Oslo University Hospital.
References
- 1 2 3 4 5 6 Lauwers, Gregory Y; Fasano, Alessio; Brown, Ian S (2015). "Duodenal lymphocytosis with no or minimal enteropathy: much ado about nothing?". Modern Pathology. 28 (S1): S22–S29. doi:10.1038/modpathol.2014.135. ISSN 0893-3952. PMID 25560597.
- 1 2 3 4 Hammer, Suntrea T. G.; Greenson, Joel K. (2013). "The Clinical Significance of Duodenal Lymphocytosis With Normal Villus Architecture". Archives of Pathology & Laboratory Medicine. 137 (9): 1216–1219. doi:10.5858/arpa.2013-0261-ra. ISSN 0003-9985. PMID 23991733.
- ↑ Shmidt, Eugenia; Smyrk, Thomas C.; Boswell, Christopher L.; Enders, Felicity T.; Oxentenko, Amy S. (2014). "Increasing duodenal intraepithelial lymphocytosis found at upper endoscopy: time trends and associations". Gastrointestinal Endoscopy. 80 (1): 105–111. doi:10.1016/j.gie.2014.01.008. ISSN 0016-5107. PMID 24565068.
- ↑ Marsh, Michael N. (1992). "Gluten, major histocompatibility complex, and the small intestine". Gastroenterology. 102 (1): 330–354. doi: 10.1016/0016-5085(92)91819-p . ISSN 0016-5085. PMID 1727768.
- 1 2 3 4 Aziz, Imran; Key, Tim; Goodwin, John G.; Sanders, David S. (2014). "Predictors for Celiac Disease in Adult Cases of Duodenal Intraepithelial Lymphocytosis". Journal of Clinical Gastroenterology. 49 (6): 477–82. doi:10.1097/mcg.0000000000000184. ISSN 0192-0790. PMID 25014240. S2CID 13090956.
- ↑ Guz-Mark, A.; Zevit, N.; Morgenstern, S.; Shamir, R. (2014-04-07). "Duodenal intraepithelial lymphocytosis is common in children without coeliac disease, and is not meaningfully influenced by Helicobacter pylori infection". Alimentary Pharmacology & Therapeutics. 39 (11): 1314–1320. doi: 10.1111/apt.12739 . ISSN 0269-2813. PMID 24702235. S2CID 22316105.
- ↑ Losurdo, Giuseppe; Piscitelli, Domenico; Giangaspero, Antonio; Principi, Mariabeatrice; Buffelli, Francesca; Giorgio, Floriana; Montenegro, Lucia; Sorrentino, Claudia; Amoruso, Annacinzia; Ierardi, Enzo; Leo, Alfredo Di (2015-06-28). "Evolution of nonspecific duodenal lymphocytosis over 2 years of follow-up". World Journal of Gastroenterology. 21 (24): 7545–52. doi: 10.3748/wjg.v21.i24.7545 . PMC 4481450 . PMID 26140001.