
William Nunn Lipscomb Jr. was a Nobel Prize-winning American inorganic and organic chemist working in nuclear magnetic resonance, theoretical chemistry, boron chemistry, and biochemistry.

A borane is a compound with the formula BxHy or a related anion. Many such boranes are known. Most common are those with 1 to 12 boron atoms. Although they have few practical applications, the boranes exhibit structures and bonding that differs strongly from the patterns seen in hydrocarbons. Hybrids of boranes and hydrocarbons, the carboranes are also well developed.

Diborane(6), commonly known as diborane, is the chemical compound with the formula B2H6. It is a toxic, colorless, and pyrophoric gas with a repulsively sweet odor. Given its simple formula, borane is a fundamental boron compound. It has attracted wide attention for its electronic structure. Several of its derivatives are useful reagents.

In chemistry, catenation is the bonding of atoms of the same element into a series, called a chain. A chain or a ring shape may be open if its ends are not bonded to each other, or closed if they are bonded in a ring. The words to catenate and catenation reflect the Latin root catena, "chain".

Decaborane, also called decaborane(14), is the borane with the chemical formula B10H14. This white crystalline compound is one of the principal boron hydride clusters, both as a reference structure and as a precursor to other boron hydrides. It is toxic and volatile, giving off a foul odor, like that of burnt rubber or chocolate.
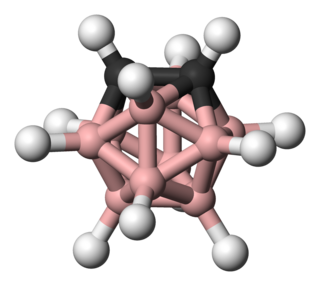
Carboranes are electron-delocalized clusters composed of boron, carbon and hydrogen atoms. Like many of the related boron hydrides, these clusters are polyhedra or fragments of polyhedra. Carboranes are one class of heteroboranes.
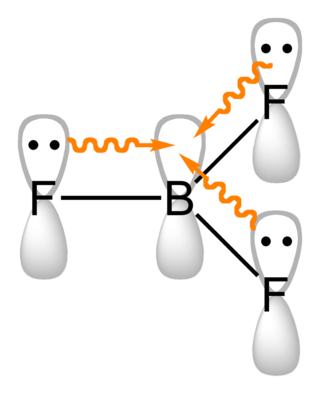
A three-center two-electron (3c–2e) bond is an electron-deficient chemical bond where three atoms share two electrons. The combination of three atomic orbitals form three molecular orbitals: one bonding, one non-bonding, and one anti-bonding. The two electrons go into the bonding orbital, resulting in a net bonding effect and constituting a chemical bond among all three atoms. In many common bonds of this type, the bonding orbital is shifted towards two of the three atoms instead of being spread equally among all three. Example molecules with 3c–2e bonds are the trihydrogen cation and diborane. In these two structures, the three atoms in each 3c-2e bond form an angular geometry, leading to a bent bond.

Organoboron chemistry or organoborane chemistry studies organoboron compounds, also called organoboranes. These chemical compounds combine boron and carbon; typically, they are organic derivatives of borane (BH3), as in the trialkyl boranes.

Borazine, also known as borazole, is an inorganic compound with the chemical formula B3H6N3. In this cyclic compound, the three BH units and three NH units alternate. The compound is isoelectronic and isostructural with benzene. For this reason borazine is sometimes referred to as “inorganic benzene”. Like benzene, borazine is a colourless liquid with an aromatic odor.
In chemistry the polyhedral skeletal electron pair theory (PSEPT) provides electron counting rules useful for predicting the structures of clusters such as borane and carborane clusters. The electron counting rules were originally formulated by Kenneth Wade, and were further developed by others including Michael Mingos; they are sometimes known as Wade's rules or the Wade–Mingos rules. The rules are based on a molecular orbital treatment of the bonding. These rules have been extended and unified in the form of the Jemmis mno rules.
Marion Frederick Hawthorne was an inorganic chemist who made contributions to the chemistry of boron hydrides, especially their clusters.
Boron compounds are compounds containing the element boron. In the most familiar compounds, boron has the formal oxidation state +3. These include oxides, sulfides, nitrides, and halides.
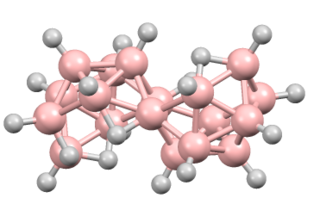
Octadecaborane is an inorganic compound, a borane with chemical formula B18H22. It is a colorless flammable solid, like many higher boron hydrides. Although the compound has no practical applications, its structure is of theoretical and pedagogical interest.

Caesium dodecaborate is an inorganic compound with the formula Cs2B12H12. It is a salt composed of caesium and dodecaborate(12) ions. The [B12H12]2− anion has been of great theoretical interest to the chemistry community.
John David Kennedy is a chemist and emeritus professor of inorganic chemistry at the University of Leeds. He works in the area of polyhedral borane chemistry.

Boron can be prepared in several crystalline and amorphous forms. Well known crystalline forms are α-rhombohedral (α-R), β-rhombohedral (β-R), and β-tetragonal (β-T). In special circumstances, boron can also be synthesized in the form of its α-tetragonal (α-T) and γ-orthorhombic (γ) allotropes. Two amorphous forms, one a finely divided powder and the other a glassy solid, are also known. Although at least 14 more allotropes have been reported, these other forms are based on tenuous evidence or have not been experimentally confirmed, or are thought to represent mixed allotropes, or boron frameworks stabilized by impurities. Whereas the β-rhombohedral phase is the most stable and the others are metastable, the transformation rate is negligible at room temperature, and thus all five phases can exist at ambient conditions. Amorphous powder boron and polycrystalline β-rhombohedral boron are the most common forms. The latter allotrope is a very hard grey material, about ten percent lighter than aluminium and with a melting point (2080 °C) several hundred degrees higher than that of steel.
In chemistry, the Jemmis mno rules represent a unified rule for predicting and systematizing structures of compounds, usually clusters. The rules involve electron counting. They were formulated by E. D. Jemmis to explain the structures of condensed polyhedral boranes such as B20H16, which are obtained by condensing polyhedral boranes by sharing a triangular face, an edge, a single vertex, or four vertices. These rules are additions and extensions to Wade's rules and polyhedral skeletal electron pair theory. The Jemmis mno rule provides the relationship between polyhedral boranes, condensed polyhedral boranes, and β-rhombohedral boron. This is similar to the relationship between benzene, condensed benzenoid aromatics, and graphite, shown by Hückel's 4n + 2 rule, as well as the relationship between tetracoordinate tetrahedral carbon compounds and diamond. The Jemmis mno rules reduce to Hückel's rule when restricted to two dimensions and reduce to Wade's rules when restricted to one polyhedron.

Carborane acidsH(CXB
11Y
5Z
6) (X, Y, Z = H, Alk, F, Cl, Br, CF3) are a class of superacids, some of which are estimated to be at least one million times stronger than 100% pure sulfuric acid in terms of their Hammett acidity function values (H0 ≤ –18) and possess computed pKa values well below –20, establishing them as some of the strongest known Brønsted acids. The best-studied example is the highly chlorinated derivative H(CHB
11Cl
11). The acidity of H(CHB
11Cl
11) was found to vastly exceed that of triflic acid, CF
3SO
3H, and bistriflimide, (CF
3SO
2)
2NH, compounds previously regarded as the strongest isolable acids.
Borane, also known as borine, is an unstable and highly reactive molecule with the chemical formula BH
3. The preparation of borane carbonyl, BH3(CO), played an important role in exploring the chemistry of boranes, as it indicated the likely existence of the borane molecule. However, the molecular species BH3 is a very strong Lewis acid. Consequently, it is highly reactive and can only be observed directly as a continuously produced, transitory, product in a flow system or from the reaction of laser ablated atomic boron with hydrogen. It normally dimerizes to diborane in the absence of other chemicals.

ortho-Carborane is the organoboron compound with the formula C2B10H12. The prefix ortho is derived from ortho. It is the most prominent carborane. This derivative has been considered for a wide range of applications from heat-resistant polymers to medical applications. It is a colorless solid that melts, without decomposition, at 320 °C.












