Antioxidants are compounds that inhibit oxidation, a chemical reaction that can produce free radicals. Autoxidation leads to degradation of organic compounds, including living matter. Antioxidants are frequently added to industrial products, such as polymers, fuels, and lubricants, to extend their usable lifetimes. Food are also treated with antioxidants to forestall spoilage, in particular the rancidification of oils and fats. In cells, antioxidants such as glutathione, mycothiol or bacillithiol, and enzyme systems like superoxide dismutase, can prevent damage from oxidative stress.
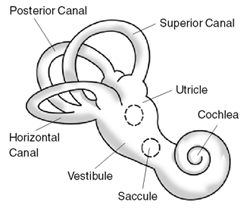
Ménière's disease (MD) is a disease of the inner ear that is characterized by potentially severe and incapacitating episodes of vertigo, tinnitus, hearing loss, and a feeling of fullness in the ear. Typically, only one ear is affected initially, but over time, both ears may become involved. Episodes generally last from 20 minutes to a few hours. The time between episodes varies. The hearing loss and ringing in the ears can become constant over time.

Peroxidases or peroxide reductases are a large group of enzymes which play a role in various biological processes. They are named after the fact that they commonly break up peroxides.
Tinnitus is a variety of sound that is heard when no corresponding external sound is present. Nearly everyone experiences faint "normal tinnitus" in a completely quiet room; but it is of concern only if it is bothersome, interferes with normal hearing, or is associated with other problems. The word tinnitus comes from the Latin tinnire, "to ring". In some people, it interferes with concentration, and can be associated with anxiety and depression.

Glutathione peroxidase (GPx) is the general name of an enzyme family with peroxidase activity whose main biological role is to protect the organism from oxidative damage. The biochemical function of glutathione peroxidase is to reduce lipid hydroperoxides to their corresponding alcohols and to reduce free hydrogen peroxide to water.
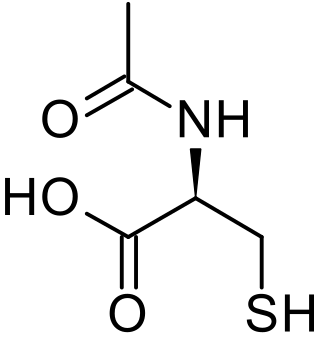
Acetylcysteine, also known as N-acetylcysteine (NAC), is a medication that is used to treat paracetamol overdose and to loosen thick mucus in individuals with chronic bronchopulmonary disorders like pneumonia and bronchitis. It has been used to treat lactobezoar in infants. It can be taken intravenously, by mouth, or inhaled as a mist. Some people use it as a dietary supplement.

Glutathione disulfide (GSSG) is a disulfide derived from two glutathione molecules.

Gliotoxin is a sulfur-containing mycotoxin that belongs to a class of naturally occurring 2,5-diketopiperazines produced by several species of fungi, especially those of marine origin. It is the most prominent member of the epipolythiopiperazines, a large class of natural products featuring a diketopiperazine with di- or polysulfide linkage. These highly bioactive compounds have been the subject of numerous studies aimed at new therapeutics. Gliotoxin was originally isolated from Gliocladium fimbriatum, and was named accordingly. It is an epipolythiodioxopiperazine metabolite that is one of the most abundantly produced metabolites in human invasive Aspergillosis (IA).
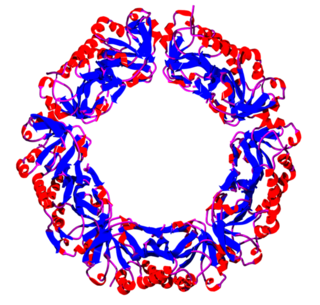
Peroxiredoxins are a ubiquitous family of antioxidant enzymes that also control cytokine-induced peroxide levels and thereby mediate signal transduction in mammalian cells. The family members in humans are PRDX1, PRDX2, PRDX3, PRDX4, PRDX5, and PRDX6. The physiological importance of peroxiredoxins is indicated by their relative abundance. Their function is the reduction of peroxides, specifically hydrogen peroxide, alkyl hydroperoxides, and peroxynitrite.

Glutathione peroxidase 1, also known as GPx1, is an enzyme that in humans is encoded by the GPX1 gene on chromosome 3. This gene encodes a member of the glutathione peroxidase family. Glutathione peroxidase functions in the detoxification of hydrogen peroxide, and is one of the most important antioxidant enzymes in humans.
In enzymology, a phospholipid-hydroperoxide glutathione peroxidase (EC 1.11.1.12) is an enzyme that catalyzes the chemical reaction

Glutathione peroxidase 4, also known as GPX4, is an enzyme that in humans is encoded by the GPX4 gene. GPX4 is a phospholipid hydroperoxidase that protects cells against membrane lipid peroxidation.

Peroxiredoxin-6 is a protein that in humans is encoded by the PRDX6 gene. It is a member of the peroxiredoxin family of antioxidant enzymes.

Glutathione peroxidase 7 is an enzyme that in humans is encoded by the GPX7 gene.
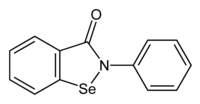
Selenium deficiency occurs when an organism lacks the required levels of selenium, a critical nutrient in many species. Deficiency, although relatively rare in healthy well-nourished individuals, can have significant negative results, affecting the health of the heart and the nervous system; contributing to depression, anxiety, and dementia; and interfering with reproduction and gestation.

Tetrandrine, a bis-benzylisoquinoline alkaloid, is a calcium channel blocker. It is isolated from the plant Stephania tetrandra, and other Chinese and Japanese herbs.

Glutathione peroxidase 3 (GPx-3), also known as plasma glutathione peroxidase (GPx-P) or extracellular glutathione peroxidase is an enzyme that in humans is encoded by the GPX3 gene.

The 3C-like protease (3CLpro) or main protease (Mpro), formally known as C30 endopeptidase or 3-chymotrypsin-like protease, is the main protease found in coronaviruses. It cleaves the coronavirus polyprotein at eleven conserved sites. It is a cysteine protease and a member of the PA clan of proteases. It has a cysteine-histidine catalytic dyad at its active site and cleaves a Gln–(Ser/Ala/Gly) peptide bond.

3CLpro-1 is an antiviral drug related to rupintrivir which acts as a 3CL protease inhibitor and was originally developed for the treatment of human enterovirus 71. It is one of the most potent of a large series of compounds developed as inhibitors of the viral enzyme 3CL protease, with an in vitroIC50 of 200 nM. It also shows activity against coronavirus diseases such as SARS and MERS, and is under investigation as a potential treatment agent for the viral disease COVID-19.
IDX-184 is an antiviral drug which was developed as a treatment for hepatitis C, acting as a NS5B RNA polymerase inhibitor. While it showed reasonable effectiveness in early clinical trials it did not progress past Phase IIb. However research using this drug has continued as it shows potentially useful activity against other emerging viral diseases such as Zika virus, and coronaviruses including MERS, and SARS-CoV-2.