See also
| Wikimedia Commons has media related to Electrokinetics . |
Electrokinetic phenomena are a family of several different effects that occur in heterogeneous fluids, or in porous bodies filled with fluid, or in a fast flow over a flat surface. The term heterogeneous here means a fluid containing particles. Particles can be solid, liquid or gas bubbles with sizes on the scale of a micrometer or nanometer. [1] [2] [3] There is a common source of all these effects—the so-called interfacial 'double layer' of charges. Influence of an external force on the diffuse layer generates tangential motion of a fluid with respect to an adjacent charged surface. This force might be electric, pressure gradient, concentration gradient, or gravity. In addition, the moving phase might be either continuous fluid or dispersed phase.
Various combinations of the driving force and moving phase determine various electrokinetic effects. According to J.Lyklema, the complete family of electrokinetic phenomena includes: [4]
There are detailed descriptions of electrokinetic phenomena in many books on interface and colloid science. [4] [5] [6] [7] [8] [3] [9] [10]
| Wikimedia Commons has media related to Electrokinetics . |

In chemistry, a colloid is a phase separated mixture in which one substance of microscopically dispersed insoluble or soluble particles is suspended throughout another substance. Sometimes the dispersed substance alone is called the colloid; the term colloidal suspension refers unambiguously to the overall mixture. Unlike a solution, whose solute and solvent constitute only one phase, a colloid has a dispersed phase and a continuous phase that arise by phase separation. Typically, colloids do not completely settle or take a long time to settle completely into two separated layers.

Electrophoresis is the motion of dispersed particles relative to a fluid under the influence of a spatially uniform electric field. Electrophoresis of positively charged particles (cations) is sometimes called cataphoresis, while electrophoresis of negatively charged particles (anions) is sometimes called anaphoresis.
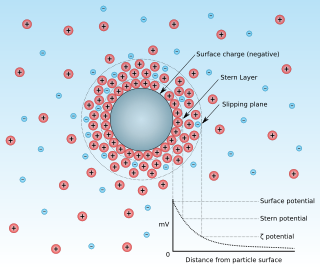
Zeta potential is the electrical potential at the slipping plane. This plane is the interface which separates mobile fluid from fluid that remains attached to the surface.
Electrohydrodynamics (EHD), also known as electro-fluid-dynamics (EFD) or electrokinetics, is the study of the dynamics of electrically charged fluids. It is the study of the motions of ionized particles or molecules and their interactions with electric fields and the surrounding fluid. The term may be considered to be synonymous with the rather elaborate electrostrictive hydrodynamics. ESHD covers the following types of particle and fluid transport mechanisms: electrophoresis, electrokinesis, dielectrophoresis, electro-osmosis, and electrorotation. In general, the phenomena relate to the direct conversion of electrical energy into kinetic energy, and vice versa.
A streaming current and streaming potential are two interrelated electrokinetic phenomena studied in the areas of surface chemistry and electrochemistry. They are an electric current or potential which originates when an electrolyte is driven by a pressure gradient through a channel or porous plug with charged walls.
Electroacoustic phenomena arise when ultrasound propagates through a fluid containing ions. The associated particle motion generates electric signals because ions have electric charge. This coupling between ultrasound and electric field is called electroacoustic phenomena. The fluid might be a simple Newtonian liquid, or complex heterogeneous dispersion, emulsion or even a porous body. There are several different electroacoustic effects depending on the nature of the fluid.
The Dukhin number is a dimensionless quantity that characterizes the contribution of the surface conductivity to various electrokinetic and electroacoustic effects, as well as to electrical conductivity and permittivity of fluid heterogeneous systems.
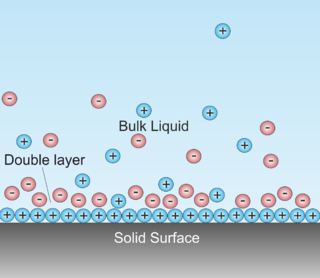
A double layer is a structure that appears on the surface of an object when it is exposed to a fluid. The object might be a solid particle, a gas bubble, a liquid droplet, or a porous body. The DL refers to two parallel layers of charge surrounding the object. The first layer, the surface charge, consists of ions adsorbed onto the object due to chemical interactions. The second layer is composed of ions attracted to the surface charge via the Coulomb force, electrically screening the first layer. This second layer is loosely associated with the object. It is made of free ions that move in the fluid under the influence of electric attraction and thermal motion rather than being firmly anchored. It is thus called the "diffuse layer".
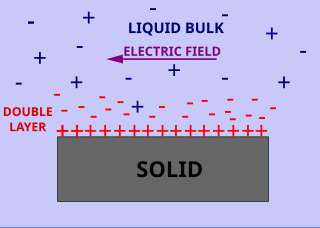
Surface conductivity is an additional conductivity of an electrolyte in the vicinity of the charged interfaces. Surface and volume conductivity of liquids correspond to the electrically driven motion of ions in an electric field. A layer of counter ions of the opposite polarity to the surface charge exists close to the interface. It is formed due to attraction of counter-ions by the surface charges. This layer of higher ionic concentration is a part of the interfacial double layer. The concentration of the ions in this layer is higher as compared to the ionic strength of the liquid bulk. This leads to the higher electric conductivity of this layer.

Interface and colloid science is an interdisciplinary intersection of branches of chemistry, physics, nanoscience and other fields dealing with colloids, heterogeneous systems consisting of a mechanical mixture of particles between 1 nm and 1000 nm dispersed in a continuous medium. A colloidal solution is a heterogeneous mixture in which the particle size of the substance is intermediate between a true solution and a suspension, i.e. between 1–1000 nm. Smoke from a fire is an example of a colloidal system in which tiny particles of solid float in air. Just like true solutions, colloidal particles are small and cannot be seen by the naked eye. They easily pass through filter paper. But colloidal particles are big enough to be blocked by parchment paper or animal membrane.

Colloid vibration current is an electroacoustic phenomenon that arises when ultrasound propagates through a fluid that contains ions and either solid particles or emulsion droplets.
Electric sonic amplitude is an electroacoustic phenomenon that is the reverse to colloid vibration current. It occurs in colloids, emulsions and other heterogeneous fluids under the influence of an oscillating electric field. This field moves particles relative to the liquid, which generates ultrasound.
The ion vibration current (IVI) and the associated ion vibration potential is an electric signal that arises when an acoustic wave propagates through a homogeneous fluid.
The streaming vibration current (SVI) and the associated streaming vibration potential is an electric signal that arises when an acoustic wave propagates through a porous body in which the pores are filled with fluid.
Sedimentation potential occurs when dispersed particles move under the influence of either gravity or centrifugation in a medium. This motion disrupts the equilibrium symmetry of the particle's double layer. While the particle moves, the ions in the electric double layer lag behind due to the liquid flow. This causes a slight displacement between the surface charge and the electric charge of the diffuse layer. As a result, the moving particle creates a dipole moment. The sum of all of the dipoles generates an electric field which is called sedimentation potential. It can be measured with an open electrical circuit, which is also called sedimentation current.

Diffusiophoresis is the spontaneous motion of colloidal particles or molecules in a fluid, induced by a concentration gradient of a different substance. In other words, it is motion of one species, A, in response to a concentration gradient in another species, B. Typically, A is colloidal particles which are in aqueous solution in which B is a dissolved salt such as sodium chloride, and so the particles of A are much larger than the ions of B. But both A and B could be polymer molecules, and B could be a small molecule. For example, concentration gradients in ethanol solutions in water move 1 μm diameter colloidal particles with diffusiophoretic velocities of order 0.1 to 1 μm/s, the movement is towards regions of the solution with lower ethanol concentration. Both species A and B will typically be diffusing but diffusiophoresis is distinct from simple diffusion: in simple diffusion a species A moves down a gradient in its own concentration.

Particle size is a notion introduced for comparing dimensions of solid particles (flecks), liquid particles (droplets), or gaseous particles (bubbles). The notion of particle size applies to colloidal particles, particles in ecology, particles present in granular material, and particles that form a granular material.
Electrodiffusiophoresis is a motion of particles dispersed in liquid induced by external homogeneous electric field, which makes it similar to electrophoresis.
Dispersion Technology Inc is a scientific instrument manufacturer located in Bedford Hills, New York. It was founded in 1996 by Philip Goetz and Dr. Andrei Dukhin. The company develops and sells analytical instruments intended for characterizing concentrated dispersions and emulsions, complying with the International Standards for acoustic particle sizing ISO 20998 and Electroacoustic zeta potential measurement ISO 13099.

Induced-charge electrokinetics in physics is the electrically driven fluid flow and particle motion in a liquid electrolyte. Consider a metal particle in contact with an aqueous solution in a chamber/channel. If different voltages apply to the end of this chamber/channel, electric field will generate in this chamber/channel. This applied electric field passes through this metal particle and causes the free charges inside the particle migrate under the skin of particle. As a result of this migration, the negative charges moves to the side which is close to the positive voltage while the positive charges moves to the opposite side of the particle. These charges under the skin of conducting particle attract the counter-ions of the aqueous solution; thus, the electric double layer (EDL) forms around the particle. The EDL sing on the surface of the conducting particle changes from positive to negative and the distribution of the charges varies along the particle geometry. Due to these variations, the EDL is non-uniform and has different sings. Thus, the induced zeta potential around the particle, and consequently slip velocity on the surface of the particle, vary as a function of local electric field. Differences in magnitude and direction of slip velocity on the surface of the conducting particle effects the flow pattern around this particle and causes micro vortices. Yasaman Daghighi and Dongqing Li, for the first time, experimentally illustrated these induced vortices around a 1.2mm diameter carbon-steel sphere under the 40V/cm direct current (DC) external electric filed. Chenhui Peng et al. also experimentally showed the patterns of electro-osmotic flow around an Au sphere when alternating current (AC) is involved . Electrokinetics here refers to a branch of science related to the motion and reaction of charged particles to the applied electric filed and its effects on its environment. It is sometimes referred as non-linear electrokinetic phenomena as well.