
Chlorine is a chemical element with the symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity on the revised Pauling scale, behind only oxygen and fluorine.

Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference, as a measurable and quantitative phenomenon, and identifiable chemical change, with the potential difference as an outcome of a particular chemical change, or vice versa. These reactions involve electrons moving via an electronically-conducting phase between electrodes separated by an ionically conducting and electronically insulating electrolyte.

In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from naturally occurring sources such as ores using an electrolytic cell. The voltage that is needed for electrolysis to occur is called the decomposition potential. The word "lysis" means to separate or break, so in terms, electrolysis would mean "breakdown via electricity".

Sodium hypochlorite is an inorganic chemical compound with the formula NaOCl, comprising a sodium cation and a hypochlorite anion. It may also be viewed as the sodium salt of hypochlorous acid. The anhydrous compound is unstable and may decompose explosively. It can be crystallized as a pentahydrate NaOCl·5H
2O, a pale greenish-yellow solid which is not explosive and is stable if kept refrigerated.

An electrolytic cell is an electrochemical cell that utilizes an external source of electrical energy to force a chemical reaction that would otherwise not occur. The external energy source is a voltage applied between the cell′s two electrodes; an anode and a cathode, which are immersed in an electrolyte solution. This is in contrast to a galvanic cell, which itself is a source of electrical energy and the foundation of a battery. The net reaction taking place in a galvanic cell is a spontaneous reaction, i.e, the Gibbs free energy remains -ve, while the net reaction taking place in an electrolytic cell is the reverse of this spontaneous reaction, i.e, the Gibbs free energy is +ve.

Hypochlorous acid is a weak acid that forms when chlorine dissolves in water, and itself partially dissociates, forming hypochlorite, ClO−. HClO and ClO− are oxidizers, and the primary disinfection agents of chlorine solutions. HClO cannot be isolated from these solutions due to rapid equilibration with its precursor, chlorine.

In chemistry, hypochlorite is an anion with the chemical formula ClO−. It combines with a number of cations to form hypochlorite salts. Common examples include sodium hypochlorite and calcium hypochlorite. The Cl-O distance in ClO− is 1.69 Å.
The chloralkali process is an industrial process for the electrolysis of sodium chloride (NaCl) solutions. It is the technology used to produce chlorine and sodium hydroxide, which are commodity chemicals required by industry. Thirty five million tons of chlorine were prepared by this process in 1987. The chlorine and sodium hydroxide produced in this process are widely used in the chemical industry.

Sodium chlorate is an inorganic compound with the chemical formula NaClO3. It is a white crystalline powder that is readily soluble in water. It is hygroscopic. It decomposes above 300 °C to release oxygen and leaves sodium chloride. Several hundred million tons are produced annually, mainly for applications in bleaching pulp to produce high brightness paper.

Electrowinning, also called electroextraction, is the electrodeposition of metals from their ores that have been put in solution via a process commonly referred to as leaching. Electrorefining uses a similar process to remove impurities from a metal. Both processes use electroplating on a large scale and are important techniques for the economical and straightforward purification of non-ferrous metals. The resulting metals are said to be electrowon.
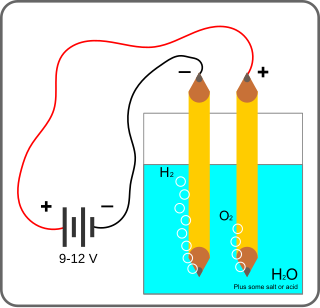
Electrolysis of water, also known as electrochemical water splitting, is the process of using electricity to decompose water into oxygen and hydrogen gas by electrolysis. Hydrogen gas released in this way can be used as hydrogen fuel, or remixed with the oxygen to create oxyhydrogen gas, which is used in welding and other applications.
Salt water chlorination is a process that uses dissolved salt for the chlorination of swimming pools and hot tubs. The chlorine generator uses electrolysis in the presence of dissolved salt to produce chlorine gas or its dissolved forms, hypochlorous acid and sodium hypochlorite, which are already commonly used as sanitizing agents in pools. Hydrogen is produced as byproduct too.
Monochloramine, often called chloramine, is the chemical compound with the formula NH2Cl. Together with dichloramine (NHCl2) and nitrogen trichloride (NCl3), it is one of the three chloramines of ammonia. It is a colorless liquid at its melting point of −66 °C (−87 °F), but it is usually handled as a dilute aqueous solution, in which form it is sometimes used as a disinfectant. Chloramine is too unstable to have its boiling point measured.
Calcium chlorate is the calcium salt of chloric acid, with the chemical formula Ca(ClO3)2. Like other chlorates, it is a strong oxidizer.
The Castner–Kellner process is a method of electrolysis on an aqueous alkali chloride solution to produce the corresponding alkali hydroxide, invented by American Hamilton Castner and Austrian Carl Kellner in the 1890s.
Bleaching of wood pulp is the chemical processing of wood pulp to lighten its color and whiten the pulp. The primary product of wood pulp is paper, for which whiteness is an important characteristic. These processes and chemistry are also applicable to the bleaching of non-wood pulps, such as those made from bamboo or kenaf.
Chlorine gas can be produced by extracting from natural materials, including the electrolysis of a sodium chloride solution (brine) and other ways.

Water chlorination is the process of adding chlorine or chlorine compounds such as sodium hypochlorite to water. This method is used to kill bacteria, viruses and other microbes in water. In particular, chlorination is used to prevent the spread of waterborne diseases such as cholera, dysentery, and typhoid.
Potassium hypochlorite is the potassium salt of hypochlorous acid. It is used in variable concentrations, often diluted in water solution. It has a light grey color and a strong chlorine smell. It can be used as a disinfectant.
Mixed oxidant solution is a type of disinfectant which is used for disinfecting, sterilization and eliminating pathogenic microorganisms in water and in many other applications. Using a mixed oxidant solution for water disinfection, compared to other methods, may have various benefits such as higher disinfecting power, stable residual chlorine in water, improved taste and smell, elimination of biofilm, and safety. A mixed-oxidant solution is produced by electrolysis of sodium chloride and is a mixture of disinfecting compounds. The main component of this product is chlorine and its derivatives (ClO−, HClO and Cl2 solution). It may also contain high amounts of chlorine dioxide solution, dissolved ozone, hydrogen peroxide, and oxygen, from which the name "mixed oxidant" is derived.










