Related Research Articles
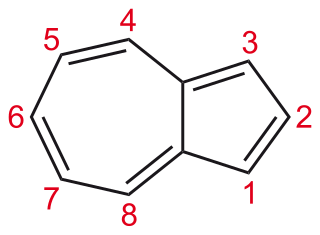
Azulene is an organic compound and an isomer of naphthalene. Naphthalene is colourless, whereas azulene is dark blue. The compound is named after its colour, as "azul" is Spanish for blue. Two terpenoids, vetivazulene (4,8-dimethyl-2-isopropylazulene) and guaiazulene (1,4-dimethyl-7-isopropylazulene), that feature the azulene skeleton are found in nature as constituents of pigments in mushrooms, guaiac wood oil, and some marine invertebrates.
A transition metal carbene complex is an organometallic compound featuring a divalent organic ligand. The divalent organic ligand coordinated to the metal center is called a carbene. Carbene complexes for almost all transition metals have been reported. Many methods for synthesizing them and reactions utilizing them have been reported. The term carbene ligand is a formalism since many are not derived from carbenes and almost none exhibit the reactivity characteristic of carbenes. Described often as M=CR2, they represent a class of organic ligands intermediate between alkyls (−CR3) and carbynes (≡CR). They feature in some catalytic reactions, especially alkene metathesis, and are of value in the preparation of some fine chemicals.
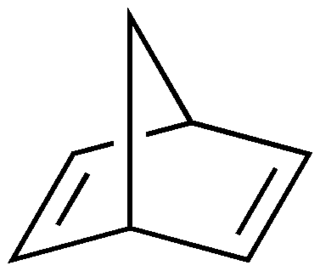
Norbornadiene is an organic compound and a bicyclic hydrocarbon. Norbornadiene is of interest as a metal-binding ligand, whose complexes are useful for homogeneous catalysis. It has been intensively studied owing to its high reactivity and distinctive structural property of being a diene that cannot isomerize. Norbornadiene is also a useful dienophile in Diels-Alder reactions.

Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe chemistry. In the Mond process, nickel tetracarbonyl is used to produce pure nickel. In organometallic chemistry, metal carbonyls serve as precursors for the preparation of other organometallic complexes.

Dicobalt octacarbonyl is an organocobalt compound with composition Co2(CO)8. This metal carbonyl is used as a reagent and catalyst in organometallic chemistry and organic synthesis, and is central to much known organocobalt chemistry. It is the parent member of a family of hydroformylation catalysts. Each molecule consists of two cobalt atoms bound to eight carbon monoxide ligands, although multiple structural isomers are known. Some of the carbonyl ligands are labile.
In chemistry, a (redox) non-innocent ligand is a ligand in a metal complex where the oxidation state is not clear. Typically, complexes containing non-innocent ligands are redox active at mild potentials. The concept assumes that redox reactions in metal complexes are either metal or ligand localized, which is a simplification, albeit a useful one.

In coordination chemistry, the bite angle is the angle on a central atom between two bonds to a bidentate ligand. This ligand–metal–ligand geometric parameter is used to classify chelating ligands, including those in organometallic complexes. It is most often discussed in terms of catalysis, as changes in bite angle can affect not just the activity and selectivity of a catalytic reaction but even allow alternative reaction pathways to become accessible.
1-Naphthol, or α-naphthol, is a fluorescent organic compound with the formula C10H7OH. It is a white solid. It is an isomer of 2-naphthol differing by the location of the hydroxyl group on the naphthalene ring. The naphthols are naphthalene homologues of phenol, with the hydroxyl group being more reactive than in the phenols. Both isomers are soluble in simple alcohols, ethers, and chloroform. They are precursors to a variety of useful compounds. Naphthols are used as biomarkers for livestock and humans exposed to polycyclic aromatic hydrocarbons.

Organocobalt chemistry is the chemistry of organometallic compounds containing a carbon to cobalt chemical bond. Organocobalt compounds are involved in several organic reactions and the important biomolecule vitamin B12 has a cobalt-carbon bond. Many organocobalt compounds exhibit useful catalytic properties, the preeminent example being dicobalt octacarbonyl.

Rhodocene is a chemical compound with the formula [Rh(C5H5)2]. Each molecule contains an atom of rhodium bound between two planar aromatic systems of five carbon atoms known as cyclopentadienyl rings in a sandwich arrangement. It is an organometallic compound as it has (haptic) covalent rhodium–carbon bonds. The [Rh(C5H5)2] radical is found above 150 °C (302 °F) or when trapped by cooling to liquid nitrogen temperatures (−196 °C [−321 °F]). At room temperature, pairs of these radicals join via their cyclopentadienyl rings to form a dimer, a yellow solid.
Binary compounds of hydrogen are binary chemical compounds containing just hydrogen and one other chemical element. By convention all binary hydrogen compounds are called hydrides even when the hydrogen atom in it is not an anion. These hydrogen compounds can be grouped into several types.
Transition metal carbyne complexes are organometallic compounds with a triple bond between carbon and the transition metal. This triple bond consists of a σ-bond and two π-bonds. The HOMO of the carbyne ligand interacts with the LUMO of the metal to create the σ-bond. The two π-bonds are formed when the two HOMO orbitals of the metal back-donate to the LUMO of the carbyne. They are also called metal alkylidynes—the carbon is a carbyne ligand. Such compounds are useful in organic synthesis of alkynes and nitriles. They have been the focus on much fundamental research.
Metal acetylacetonates are coordination complexes derived from the acetylacetonate anion (CH
3COCHCOCH−
3) and metal ions, usually transition metals. The bidentate ligand acetylacetonate is often abbreviated acac. Typically both oxygen atoms bind to the metal to form a six-membered chelate ring. The simplest complexes have the formula M(acac)3 and M(acac)2. Mixed-ligand complexes, e.g. VO(acac)2, are also numerous. Variations of acetylacetonate have also been developed with myriad substituents in place of methyl (RCOCHCOR′−). Many such complexes are soluble in organic solvents, in contrast to the related metal halides. Because of these properties, acac complexes are sometimes used as catalyst precursors and reagents. Applications include their use as NMR "shift reagents" and as catalysts for organic synthesis, and precursors to industrial hydroformylation catalysts. C
5H
7O−
2 in some cases also binds to metals through the central carbon atom; this bonding mode is more common for the third-row transition metals such as platinum(II) and iridium(III).
The phosphaethynolate anion, also referred to as PCO, is the phosphorus-containing analogue of the cyanate anion with the chemical formula [PCO]− or [OCP]−. The anion has a linear geometry and is commonly isolated as a salt. When used as a ligand, the phosphaethynolate anion is ambidentate in nature meaning it forms complexes by coordinating via either the phosphorus or oxygen atoms. This versatile character of the anion has allowed it to be incorporated into many transition metal and actinide complexes but now the focus of the research around phosphaethynolate has turned to utilising the anion as a synthetic building block to organophosphanes.
Heterobimetallic catalysis is an approach to catalysis that employs two different metals to promote a chemical reaction. Included in this definition are cases where: 1) each metal activates a different substrate, 2) both metals interact with the same substrate, and 3) only one metal directly interacts with the substrate(s), while the second metal interacts with the first.

Transition metal pyridine complexes encompass many coordination complexes that contain pyridine as a ligand. Most examples are mixed-ligand complexes. Many variants of pyridine are also known to coordinate to metal ions, such as the methylpyridines, quinolines, and more complex rings.

Hexaphosphabenzene is a valence isoelectronic analogue of benzene and is expected to have a similar planar structure due to resonance stabilization. Although several other allotropes of phosphorus are stable, no evidence for the existence of P6 has been reported. Preliminary ab initio calculations on the trimerisation of P2 leading to the formation of the cyclic P6 were performed, and it was predicted that hexaphosphabenzene would decompose to free P2 with an energy barrier of 13−15.4 kcal mol−1, and would therefore not be observed in the uncomplexed state under normal experimental conditions. The presence of an added solvent, such as ethanol, might lead to the formation of intermolecular hydrogen bonds which may block the destabilizing interaction between phosphorus lone pairs and consequently stabilize P6. The moderate barrier suggests that hexaphosphabenzene could be synthesized from a [2+2+2] cycloaddition of three P2 molecules. Currently, this is a synthetic endeavour which remains to be conquered.

Transition metal dithiocarbamate complexes are coordination complexes containing one or more dithiocarbamate ligand, which are typically abbreviated R2dtc−. Many complexes are known. Several homoleptic derivatives have the formula M(R2dtc)n where n = 2 and 3.

In organometallic chemistry, transition metal complexes of nitrite describes families of coordination complexes containing one or more nitrite ligands. Although the synthetic derivatives are only of scholarly interest, metal-nitrite complexes occur in several enzymes that participate in the nitrogen cycle.
Cobalt compounds are chemical compounds formed by cobalt with other elements. In the compound, the most stable oxidation state of cobalt is the +2 oxidation state, and in the presence of specific ligands, there are also stable compounds with +3 valence. In addition, there are cobalt compounds in high oxidation states +4, +5 and low oxidation states -1, 0, +1.
References
- ↑ Bally, T. (2010). "Isomerism: The same but different" (PDF). Nature Chemistry. 2 (3): 165–166. Bibcode:2010NatCh...2..165B. doi:10.1038/nchem.564. PMID 21124473.
- ↑ Evangelio, E.; Ruiz-Molina, D. (2005). "Valence Tautomerism: New Challenges for Electroactive Ligands". European Journal of Inorganic Chemistry. 2005 (15): 2957. doi:10.1002/ejic.200500323.
- ↑ Jones, L. W. (1917). "Electromerism, A Case of Chemical Isomerism Resulting from a Difference in Distribution of Valence Electrons". Science. 46 (1195): 493–502. Bibcode:1917Sci....46..493J. doi:10.1126/science.46.1195.493. PMID 17818241.
- ↑ Buchanan, R. M.; Pierpont, C. G. (1980). "Tautomeric catecholate-semiquinone interconversion via metal-ligand electron transfer. Structural, spectral, and magnetic properties of (3,5-di-tert-butylcatecholato)(3,5-di-tert-butylsemiquinone)(bipyridyl)cobalt(III), a complex containing mixed-valence organic ligands". Journal of the American Chemical Society. 102 (15): 4951. doi:10.1021/ja00535a021.
- ↑ Puschmann, F.; Harmer, J.; Stein, D.; Rüegger, H.; De Bruin, B.; Grützmacher, H. (2010). "Electromeric rhodium radical complexes". Angewandte Chemie International Edition in English. 49 (2): 385–389. doi:10.1002/anie.200903201. PMID 19957252.
- ↑ Puschmann, F.F.; Grützmacher, H.; de Bruin, B. (2010). "Rhodium(0) Metalloradicals in Binuclear C−H Activation". Journal of the American Chemical Society . 132 (1): 73–75. doi:10.1021/ja909022p. PMID 20000835.
- ↑ Müller, B.; Bally, T.; Gerson, F.; De Meijere, A.; Von Seebach, M. (2003). ""Electromers" of the tetramethyleneethane radical cation and their nonexistence in the octamethyl derivative: interplay of experiment and theory". Journal of the American Chemical Society. 125 (45): 13776–13783. doi:10.1021/ja037252v. PMID 14599217.
- ↑ Jiang, Quan; Cundari, Thomas R. (2020-10-22). "DFT Calculations Investigate Competing Pathways to Form Dimeric Neopentylpalladium(II) Amido Complexes: The Critical Importance of Dispersion". The Journal of Physical Chemistry A. 124 (42): 8798–8805. Bibcode:2020JPCA..124.8798J. doi:10.1021/acs.jpca.0c08102. ISSN 1089-5639. PMID 33026808.
- ↑ Jiang, Quan; Cundari, Thomas (2020-08-13). "DFT Calculations Investigate Competing Pathways to Form Dimeric Neopentylpalladium(II) Amido Complexes". doi:10.26434/chemrxiv.11914377.v2.
{{cite journal}}: Cite journal requires|journal=(help)