Related Research Articles
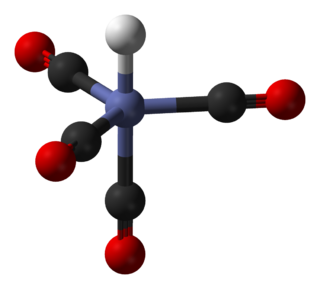
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs, often through Lewis bases. The nature of metal–ligand bonding can range from covalent to ionic. Furthermore, the metal–ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acidic "ligands".

Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large proportion of all proteins are part of this category. For instance, at least 1000 human proteins contain zinc-binding protein domains although there may be up to 3000 human zinc metalloproteins.

Plastocyanin is a copper-containing protein that mediates electron-transfer. It is found in a variety of plants, where it participates in photosynthesis. The protein is a prototype of the blue copper proteins, a family of intensely blue-colored metalloproteins. Specifically, it falls into the group of small type I blue copper proteins called "cupredoxins".
Cuprate loosely refers to a material that can be viewed as containing anionic copper complexes. Examples include tetrachloridocuprate ([CuCl4]2−), the superconductor YBa2Cu3O7, and the organocuprates (e.g., dimethylcuprate [Cu(CH3)2]−). The term cuprates derives from the Latin word for copper, cuprum. The term is mainly used in three contexts: oxide materials, anionic coordination complexes, and anionic organocopper compounds.
Copper proteins are proteins that contain one or more copper ions as prosthetic groups. Copper proteins are found in all forms of air-breathing life. These proteins are usually associated with electron-transfer with or without the involvement of oxygen (O2). Some organisms even use copper proteins to carry oxygen instead of iron proteins. A prominent copper proteins in humans is in cytochrome c oxidase (cco). The enzyme cco mediates the controlled combustion that produces ATP.
Catechol oxidase is a copper oxidase that contains a type 3 di-copper cofactor and catalyzes the oxidation of ortho-diphenols into ortho-quinones coupled with the reduction of molecular oxygen to water. It is present in a variety of species of plants and fungi including Ipomoea batatas and Camellia sinensis. Metalloenzymes with type 3 copper centers are characterized by their ability to reversibly bind dioxygen at ambient conditions. In plants, catechol oxidase plays a key role in enzymatic browning by catalyzing the oxidation of catechol to o-quinone in the presence of oxygen, which can rapidly polymerize to form the melanin that grants damaged fruits their dark brown coloration.
Nitrite reductase refers to any of several classes of enzymes that catalyze the reduction of nitrite. There are two classes of NIR's. A multi haem enzyme reduces NO2− to a variety of products. Copper containing enzymes carry out a single electron transfer to produce nitric oxide.

Organocopper chemistry is the study of the physical properties, reactions, and synthesis of organocopper compounds, which are organometallic compounds containing a carbon to copper chemical bond. They are reagents in organic chemistry.
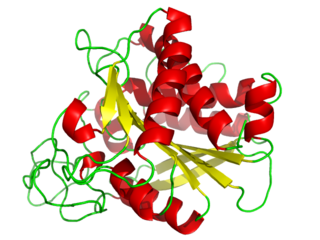
Carboxypeptidase A usually refers to the pancreatic exopeptidase that hydrolyzes peptide bonds of C-terminal residues with aromatic or aliphatic side-chains. Most scientists in the field now refer to this enzyme as CPA1, and to a related pancreatic carboxypeptidase as CPA2.
In organometallic chemistry, a migratory insertion is a type of reaction wherein two ligands on a metal complex combine. It is a subset of reactions that very closely resembles the insertion reactions, and both are differentiated by the mechanism that leads to the resulting stereochemistry of the products. However, often the two are used interchangeably because the mechanism is sometimes unknown. Therefore, migratory insertion reactions or insertion reactions, for short, are defined not by the mechanism but by the overall regiochemistry wherein one chemical entity interposes itself into an existing bond of typically a second chemical entity e.g.:

Azurin is a small, periplasmic, bacterial blue copper protein found in Pseudomonas, Bordetella, or Alcaligenes bacteria. Azurin moderates single-electron transfer between enzymes associated with the cytochrome chain by undergoing oxidation-reduction between Cu(I) and Cu(II). Each monomer of an azurin tetramer has a molecular weight of approximately 14kDa, contains a single copper atom, is intensively blue, and has a fluorescence emission band centered at 308 nm.
Zinc compounds are chemical compounds containing the element zinc which is a member of the group 12 of the periodic table. The oxidation state of zinc in most compounds is the group oxidation state of +2. Zinc may be classified as a post-transition main group element with zinc(II). Zinc compounds are noteworthy for their nondescript behavior, they are generally colorless, do not readily engage in redox reactions, and generally adopt symmetrical structures.
Cyanometallates or cyanometalates are a class of coordination compounds, most often consisting only of cyanide ligands. Most are anions. Cyanide is a highly basic and small ligand, hence it readily saturates the coordination sphere of metal ions. The resulting cyanometallate anions are often used as building blocks for more complex structures called coordination polymers, the best known example of which is Prussian blue, a common dyestuff.

A metal salen complex is a coordination compound between a metal cation and a ligand derived from N,N′-bis(salicylidene)ethylenediamine, commonly called salen. The classical example is salcomine, the complex with divalent cobalt Co2+, usually denoted as Co(salen). These complexes are widely investigated as catalysts and enzyme mimics.

Galactose oxidase is an enzyme that catalyzes the oxidation of D-galactose in some species of fungi.

The carbonic anhydrases form a family of enzymes that catalyze the interconversion between carbon dioxide and water and the dissociated ions of carbonic acid. The active site of most carbonic anhydrases contains a zinc ion. They are therefore classified as metalloenzymes. The enzyme maintains acid-base balance and helps transport carbon dioxide.

Acetyl-CoA synthase (ACS), not to be confused with Acetyl-CoA synthetase or Acetate-CoA ligase, is a nickel-containing enzyme involved in the metabolic processes of cells. Together with Carbon monoxide dehydrogenase (CODH), it forms the bifunctional enzyme Acetyl-CoA Synthase/Carbon Monoxide Dehydrogenase (ACS/CODH) found in anaerobic organisms such as archaea and bacteria. The ACS/CODH enzyme works primarily through the Wood–Ljungdahl pathway which converts carbon dioxide to Acetyl-CoA. The recommended name for this enzyme is CO-methylating acetyl-CoA synthase.
Vicilin is a legumin-associated globulin protein. Vicilin can be described as a storage protein found in legumes such as the pea or lentil. Vicilin is a protein that protects plants from fungi and microorganism. It has been hypothesized it's an allergen in pea allergy responses.
Transition metal amino acid complexes are a large family of coordination complexes containing the conjugate bases of the amino acids, the 2-aminocarboxylates. Amino acids are prevalent in nature, and all of them function as ligands toward the transition metals. Not included in this article are complexes of the amides and ester derivatives of amino acids. Also excluded are the polyamino acids including the chelating agents EDTA and NTA.
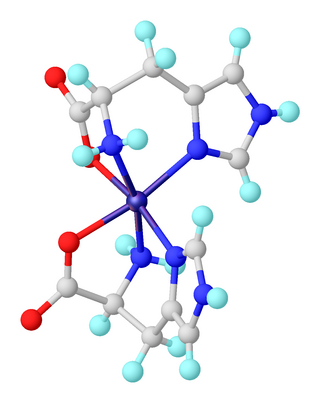
A transition metal imidazole complex is a coordination complex that has one or more imidazole ligands. Complexes of imidazole itself are of little practical importance. In contrast, imidazole derivatives, especially histidine, are pervasive ligands in biology where they bind metal cofactors.
References
- ↑ IUPAC , Compendium of Chemical Terminology , 2nd ed. (the "Gold Book") (1997). Online corrected version: (1997) " Entatic state ". doi : 10.1351/goldbook.ET06763
- ↑ Vallee, B. L.; Williams, R. J. P. (1968). "Metalloenzymes: entatic nature of their active sites". Proc. Natl. Acad. Sci. USA. 59 (2): 498–505. doi:10.1073/pnas.59.2.498.
- ↑ Lindskog, Sven; Malmström, Bo G. (1962). "Metal binding and catalytic activity in bovine carbonic anhydrase". J. Biol. Chem. 237 (4): 1129–1137.
- ↑ Perry A Frey; Adrian D Hegeman. Enzymatic Reaction Mechanisms. Oxford University Press. p. 210. ISBN 0-19-512258-5.
- ↑ Nikolas Kaltsoyannis; John E. McGrady; Jochen Autschbach (2004). Principles and Applications of Density Functional Theory in Inorganic Chemistry. Springer. pp. 47–49. ISBN 3-540-21861-0.