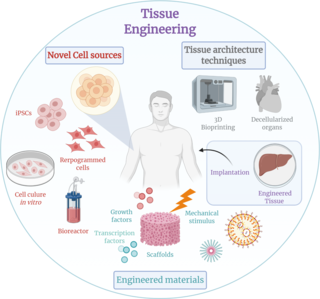
Tissue engineering is a biomedical engineering discipline that uses a combination of cells, engineering, materials methods, and suitable biochemical and physicochemical factors to restore, maintain, improve, or replace different types of biological tissues. Tissue engineering often involves the use of cells placed on tissue scaffolds in the formation of new viable tissue for a medical purpose but is not limited to applications involving cells and tissue scaffolds. While it was once categorized as a sub-field of biomaterials, having grown in scope and importance it can be considered as a field of its own.

Cultured meat is a meat produced by in vitro cell cultures of animal cells. It is a form of cellular agriculture, with such agricultural methods being explored in the context of increased consumer demand for protein.

Chinese hamster ovary (CHO) cells are an epithelial cell line derived from the ovary of the Chinese hamster, often used in biological and medical research and commercially in the production of recombinant therapeutic proteins. They have found wide use in studies of genetics, toxicity screening, nutrition and gene expression, particularly to express recombinant proteins. CHO cells are the most commonly used mammalian hosts for industrial production of recombinant protein therapeutics.

The G0 phase describes a cellular state outside of the replicative cell cycle. Classically, cells were thought to enter G0 primarily due to environmental factors, like nutrient deprivation, that limited the resources necessary for proliferation. Thus it was thought of as a resting phase. G0 is now known to take different forms and occur for multiple reasons. For example, most adult neuronal cells, among the most metabolically active cells in the body, are fully differentiated and reside in a terminal G0 phase. Neurons reside in this state, not because of stochastic or limited nutrient supply, but as a part of their developmental program.

A bioreactor refers to any manufactured device or system that supports a biologically active environment. In one case, a bioreactor is a vessel in which a chemical process is carried out which involves organisms or biochemically active substances derived from such organisms. This process can either be aerobic or anaerobic. These bioreactors are commonly cylindrical, ranging in size from litres to cubic metres, and are often made of stainless steel. It may also refer to a device or system designed to grow cells or tissues in the context of cell culture. These devices are being developed for use in tissue engineering or biochemical/bioprocess engineering.
Cell culture is the process by which cells are grown under controlled conditions, generally outside their natural environment. After the cells of interest have been isolated from living tissue, they can subsequently be maintained under carefully controlled conditions. These conditions vary for each cell type, but generally consist of a suitable vessel with a substrate or medium that supplies the essential nutrients (amino acids, carbohydrates, vitamins, minerals), growth factors, hormones, and gases (CO2, O2), and regulates the physio-chemical environment (pH buffer, osmotic pressure, temperature). Most cells require a surface or an artificial substrate to form an adherent culture as a monolayer (one single-cell thick), whereas others can be grown free floating in a medium as a suspension culture. The lifespan of most cells is genetically determined, but some cell culturing cells have been “transformed” into immortal cells which will reproduce indefinitely if the optimal conditions are provided.

A growth medium or culture medium is a solid, liquid, or semi-solid designed to support the growth of a population of microorganisms or cells via the process of cell proliferation or small plants like the moss Physcomitrella patens. Different types of media are used for growing different types of cells.
Matrigel is the trade name for the solubilized basement membrane matrix secreted by Engelbreth-Holm-Swarm (EHS) mouse sarcoma cells produced by Corning Life Sciences. Matrigel resembles the laminin/collagen IV-rich basement membrane extracellular environment found in many tissues and is used by cell biologists as a substrate for culturing cells.
Industrial fermentation is the intentional use of fermentation in manufacturing products useful to humans. In addition to the mass production of fermented foods and drinks, industrial fermentation has widespread applications in chemical industry. Commodity chemicals, such as acetic acid, citric acid, and ethanol are made by fermentation. Moreover, nearly all commercially produced industrial enzymes, such as lipase, invertase and rennet, are made by fermentation with genetically modified microbes. In some cases, production of biomass itself is the objective, as is the case for single-cell proteins, baker's yeast, and starter cultures for lactic acid bacteria used in cheesemaking.
Fed-batch culture is, in the broadest sense, defined as an operational technique in biotechnological processes where one or more nutrients (substrates) are fed (supplied) to the bioreactor during cultivation and in which the product(s) remain in the bioreactor until the end of the run. An alternative description of the method is that of a culture in which "a base medium supports initial cell culture and a feed medium is added to prevent nutrient depletion". It is also a type of semi-batch culture. In some cases, all the nutrients are fed into the bioreactor. The advantage of the fed-batch culture is that one can control concentration of fed-substrate in the culture liquid at arbitrarily desired levels.

A microcarrier is a support matrix that allows for the growth of adherent cells in bioreactors. Instead of on a flat surface, cells are cultured on the surface of spherical microcarriers so that each particle carries several hundred cells, and therefore expansion capacity can be multiplied several times over. It provides a straightforward way to scale up culture systems for industrial production of cell or protein-based therapies, or for research purposes.
Plant tissue culture is a collection of techniques used to maintain or grow plant cells, tissues or organs under sterile conditions on a nutrient culture medium of known composition. It is widely used to produce clones of a plant in a method known as micropropagation. Different techniques in plant tissue culture may offer certain advantages over traditional methods of propagation, including:
A chemically defined medium is a growth medium suitable for the in vitro cell culture of human or animal cells in which all of the chemical components are known. Standard cell culture media commonly consist of a basal medium supplemented with animal serum as a source of nutrients and other ill-defined factors. The technical disadvantages to using serum include its undefined nature, batch-to-batch variability in composition, and the risk of contamination.

A moss bioreactor is a photobioreactor used for the cultivation and propagation of mosses. It is usually used in molecular farming for the production of recombinant protein using transgenic moss. In environmental science moss bioreactors are used to multiply peat mosses e.g. by the Mossclone consortium to monitor air pollution.
A 3D cell culture is an artificially created environment in which biological cells are permitted to grow or interact with their surroundings in all three dimensions. Unlike 2D environments, a 3D cell culture allows cells in vitro to grow in all directions, similar to how they would in vivo. These three-dimensional cultures are usually grown in bioreactors, small capsules in which the cells can grow into spheroids, or 3D cell colonies. Approximately 300 spheroids are usually cultured per bioreactor.
The in vivo bioreactor is a tissue engineering paradigm that uses bioreactor methodology to grow neotissue in vivo that augments or replaces malfunctioning native tissue. Tissue engineering principles are used to construct a confined, artificial bioreactor space in vivo that hosts a tissue scaffold and key biomolecules necessary for neotissue growth. Said space often requires inoculation with pluripotent or specific stem cells to encourage initial growth, and access to a blood source. A blood source allows for recruitment of stem cells from the body alongside nutrient delivery for continual growth. This delivery of cells and nutrients to the bioreactor eventually results in the formation of a neotissue product.
A Hollow fiber bioreactor is a 3 dimensional cell culturing system based on hollow fibers, which are small, semi-permeable capillary membranes arranged in parallel array with a typical molecular weight cut-off (MWCO) range of 10-30 kDa. These hollow fiber membranes are often bundled and housed within tubular polycarbonate shells to create hollow fiber bioreactor cartridges. Within the cartridges, which are also fitted with inlet and outlet ports, are two compartments: the intracapillary (IC) space within the hollow fibers, and the extracapillary (EC) space surrounding the hollow fibers.

Cellular agriculture focuses on the production of agriculture products from cell cultures using a combination of biotechnology, tissue engineering, molecular biology, and synthetic biology to create and design new methods of producing proteins, fats, and tissues that would otherwise come from traditional agriculture. Most of the industry is focused on animal products such as meat, milk, and eggs, produced in cell culture rather than raising and slaughtering farmed livestock which is associated with substantial global problems of detrimental environmental impacts, animal welfare, food security and human health. Cellular agriculture is field of the biobased economy. The most well known cellular agriculture concept is cultured meat.
In vitro spermatogenesis is the process of creating male gametes (spermatozoa) outside of the body in a culture system. The process could be useful for fertility preservation, infertility treatment and may further develop the understanding of spermatogenesis at the cellular and molecular level.

A cell suspension or suspension culture is a type of cell culture in which single cells or small aggregates of cells are allowed to function and multiply in an agitated growth medium, thus forming a suspension. Suspension culture is one of the two classical types of cell culture, the other being adherent culture. The history of suspension cell culture closely aligns with the history of cell culture overall, but differs in maintenance methods and commercial applications. The cells themselves can either be derived from homogenized tissue or from another type of culture. Suspension cell culture is commonly used to culture nonadhesive cell lines, plant cells, and insect cells. While some cell lines are cultured in suspension, the majority of commercially available mammalian cell lines are adherent. Suspension cell cultures need to be agitated and may require specialized equipment or flasks. These cultures, like all cell cultures, need to be maintained with nutrient containing media and cultured in a specific cell density range to avoid cell death.











