Related Research Articles

An explosive is a reactive substance that contains a great amount of potential energy that can produce an explosion if released suddenly, usually accompanied by the production of light, heat, sound, and pressure. An explosive charge is a measured quantity of explosive material, which may either be composed solely of one ingredient or be a mixture containing at least two substances.
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula CH3. In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in many organic compounds. It is a very stable group in most molecules. While the methyl group is usually part of a larger molecule, bounded to the rest of the molecule by a single covalent bond, it can be found on its own in any of three forms: methanide anion, methylium cation or methyl radical. The anion has eight valence electrons, the radical seven and the cation six. All three forms are highly reactive and rarely observed.

Trinitrotoluene, more commonly known as TNT, more specifically 2,4,6-trinitrotoluene, and by its preferred IUPAC name 2-methyl-1,3,5-trinitrobenzene, is a chemical compound with the formula C6H2(NO2)3CH3. TNT is occasionally used as a reagent in chemical synthesis, but it is best known as an explosive material with convenient handling properties. The explosive yield of TNT is considered to be the standard comparative convention of bombs and asteroid impacts. In chemistry, TNT is used to generate charge transfer salts.
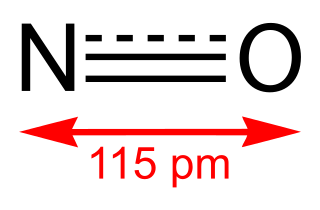
Nitric oxide is a colorless gas with the formula NO. It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its chemical formula. Nitric oxide is also a heteronuclear diatomic molecule, a class of molecules whose study spawned early modern theories of chemical bonding.

Acetone peroxide is an organic peroxide and a primary explosive. It is produced by the reaction of acetone and hydrogen peroxide to yield a mixture of linear monomer and cyclic dimer, trimer, and tetramer forms. The dimer is known as diacetone diperoxide (DADP). The trimer is known as triacetone triperoxide (TATP) or tri-cyclic acetone peroxide (TCAP). Acetone peroxide takes the form of a white crystalline powder with a distinctive bleach-like odor or a fruit-like smell when pure, and can explode powerfully if subjected to heat, friction, static electricity, concentrated sulfuric acid, strong UV radiation or shock. Until about 2015, explosives detectors were not set to detect non-nitrogenous explosives, as most explosives used preceding 2015 were nitrogen-based. TATP, being nitrogen-free, has been used as the explosive of choice in several terrorist bomb attacks since 2001.

Barium oxide, also known as baria, is a white hygroscopic non-flammable compound with the formula BaO. It has a cubic structure and is used in cathode ray tubes, crown glass, and catalysts. It is harmful to human skin and if swallowed in large quantity causes irritation. Excessive quantities of barium oxide may lead to death.

Hydrazoic acid, also known as hydrogen azide or azoimide, is a compound with the chemical formula HN3. It is a colorless, volatile, and explosive liquid at room temperature and pressure. It is a compound of nitrogen and hydrogen, and is therefore a pnictogen hydride. It was first isolated in 1890 by Theodor Curtius. The acid has few applications, but its conjugate base, the azide ion, is useful in specialized processes.

Oxygen fluorides are compounds of elements oxygen and fluorine with the general formula OnF2, where n = 1 to 6. Many different oxygen fluorides are known:
Dioxygen difluoride is a compound of fluorine and oxygen with the molecular formula O2F2. It can exist as an orange-colored solid which melts into a red liquid at −163 °C (110 K). It is an extremely strong oxidant and decomposes into oxygen and fluorine even at −160 °C (113 K) at a rate of 4% per day — its lifetime at room temperature is thus extremely short. Dioxygen difluoride reacts vigorously with nearly every chemical it encounters (including ordinary ice) leading to its onomatopoeic nickname FOOF (a play on its chemical structure and its explosive tendencies).
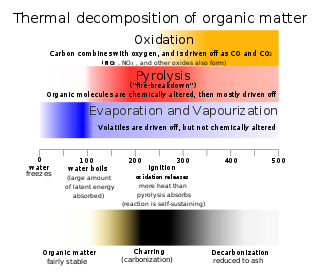
Thermal decomposition is a chemical decomposition caused by heat. The decomposition temperature of a substance is the temperature at which the substance chemically decomposes. The reaction is usually endothermic as heat is required to break chemical bonds in the compound undergoing decomposition. If decomposition is sufficiently exothermic, a positive feedback loop is created producing thermal runaway and possibly an explosion or other chemical reaction.

Azobisisobutyronitrile (abbreviated AIBN) is an organic compound with the formula [(CH3)2C(CN)]2N2. This white powder is soluble in alcohols and common organic solvents but is insoluble in water. It is often used as a foamer in plastics and rubber and as a radical initiator.

Hexamethylene triperoxide diamine (HMTD) is a high explosive organic compound. HMTD is an organic peroxide, a heterocyclic compound with a cage-like structure. It is a primary explosive. It has been considered as an initiating explosive for blasting caps in the early part of 20th century, mostly because of its high initiating power and its inexpensive production. As such, it was quickly taken up as a primary explosive in mining applications. However, it has since been superseded by more (chemically) stable compounds such as dextrinated lead azide and DDNP. HMTD is widely used in amateur-made blasting caps.

Trioxidane, also called hydrogen trioxide is an inorganic compound with the chemical formula H[O]
3H. It is one of the unstable hydrogen polyoxides. In aqueous solutions, trioxidane decomposes to form water and singlet oxygen:

Erythritol tetranitrate (ETN) is an explosive compound chemically similar to PETN, though it is thought to be slightly more sensitive to friction and impact.
Advanced oxidation processes (AOPs), in a broad sense, are a set of chemical treatment procedures designed to remove organic (and sometimes inorganic) materials in water and wastewater by oxidation through reactions with hydroxyl radicals (·OH). In real-world applications of wastewater treatment, however, this term usually refers more specifically to a subset of such chemical processes that employ ozone (O3), hydrogen peroxide (H2O2) and/or UV light.

In chemistry, dioxirane is a compound with formula CH
2O
2, whose molecule consists of a ring with one carbon and two oxygen atoms, and two hydrogen atoms attached to the carbon. It is a heterocyclic compound, the smallest cyclic organic peroxide.

An explosion is a rapid expansion in volume of a given amount of matter associated with an extreme outward release of energy, usually with the generation of high temperatures and release of high-pressure gases. Explosions may also be generated by a slower expansion that would normally not be forceful, but is not allowed to expand, so that when whatever is containing the expansion is broken by the pressure that builds as the matter inside tries to expand, the matter expands forcefully. An example of this is a volcanic eruption created by the expansion of magma in a magma chamber as it rises to the surface. Supersonic explosions created by high explosives are known as detonations and travel through shock waves. Subsonic explosions are created by low explosives through a slower combustion process known as deflagration.
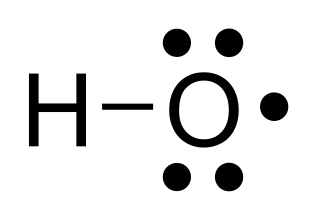
In chemistry, a radical, also known as a free radical, is an atom, molecule, or ion that has at least one unpaired valence electron. With some exceptions, these unpaired electrons make radicals highly chemically reactive. Many radicals spontaneously dimerize. Most organic radicals have short lifetimes.
Thermochemical cycles combine solely heat sources (thermo) with chemical reactions to split water into its hydrogen and oxygen components. The term cycle is used because aside of water, hydrogen and oxygen, the chemical compounds used in these processes are continuously recycled.
In situ chemical oxidation (ISCO), a form of advanced oxidation process, is an environmental remediation technique used for soil and/or groundwater remediation to lower the concentrations of targeted environmental contaminants to acceptable levels. ISCO is accomplished by introducing strong chemical oxidizers into the contaminated medium to destroy chemical contaminants in place. It can be used to remediate a variety of organic compounds, including some that are resistant to natural degradation. The in situ in ISCO is just Latin for "in place", signifying that ISCO is a chemical oxidation reaction that occurs at the site of the contamination.
References
- ↑ Dubnikova, Faina; Kosloff, Ronnie; Almog, Joseph; Zeiri, Yehuda; Boese, Roland; Itzhaky, Harel; Alt, Aaron; Keinan, Ehud (2005). "Decomposition of Triacetone Triperoxide is an Entropic Explosion". Journal of the American Chemical Society. 127 (4): 1146–59. doi:10.1021/ja0464903. PMID 15669854.
- ↑ Dubnikova, Faina; Kosloff, Ronnie; Yehuda, Zeiri (2006). Schubert, Hitmar; Kuznetsov, Audrey (eds.). Rational Detection Schemes for TATP NATO Advanced Research Workshop. Netherlands: Springer. pp. 111–112. ISBN 978-1402048869 . Retrieved 6 February 2017."The calculated thermal decomposition pathway of the TATP molecule was a complicated multistep process with several highly reactive intermediates, including singlet molecular oxygen and various biradicals. Of note, the calculations predict the formation of acetone and ozone as the main decomposition products and not the intuitively expected oxidation products. The key conclusion from this study is that the explosion of TATP is not a thermochemically highly favored event. Rather, the explosion involves entropy burst, which is the result of the formation of 4 gas-phase molecules from every molecule of TATP in the solid state. Quite unexpectedly, the 3 isopropylidene units of the TATP molecule do not play the role of fuel that can be oxidized and release energy during the explosion. Instead, these units function as molecular scaffolds that hold the 3 peroxide units close together spatially in the appropriate orientation for the decomposition chain reaction."
- ↑ "In the Media | Scripps Research". Archived from the original on November 5, 2005. Retrieved July 17, 2005.
- ↑ "Archived copy". Archived from the original on 2005-02-15. Retrieved 2005-07-17.
{{cite web}}: CS1 maint: archived copy as title (link)[ full citation needed ] - ↑ "Archived copy" (PDF). Archived from the original (PDF) on 2011-05-14. Retrieved 2005-07-17.
{{cite web}}: CS1 maint: archived copy as title (link)[ full citation needed ] - ↑ Sinditskii, V.P; Kolesov, V.I; Egorshev, V.Yu; Patrikeev, D.I; Dorofeeva, O.V (2014). "Thermochemistry of cyclic acetone peroxides". Thermochimica Acta. 585: 10–15. doi:10.1016/j.tca.2014.03.046.
- ↑ Van Duin, Adri C T; Zeiri, Yehuda; Dubnikova, Faina; Kosloff, Ronnie; Goddard, William A (2005). "Atomistic-Scale Simulations of the Initial Chemical Events in the Thermal Initiation of Triacetonetriperoxide". Journal of the American Chemical Society. 127 (31): 11053–62. doi:10.1021/ja052067y. PMID 16076213.