Estrone (E1), also spelled oestrone, is a steroid, a weak estrogen, and a minor female sex hormone. It is one of three major endogenous estrogens, the others being estradiol and estriol. Estrone, as well as the other estrogens, are synthesized from cholesterol and secreted mainly from the gonads, though they can also be formed from adrenal androgens in adipose tissue. Relative to estradiol, both estrone and estriol have far weaker activity as estrogens. Estrone can be converted into estradiol, and serves mainly as a precursor or metabolic intermediate of estradiol. It is both a precursor and metabolite of estradiol.

Estriol (E3), also spelled oestriol, is a steroid, a weak estrogen, and a minor female sex hormone. It is one of three major endogenous estrogens, the others being estradiol and estrone. Levels of estriol in women who are not pregnant are almost undetectable. However, during pregnancy, estriol is synthesized in very high quantities by the placenta and is the most produced estrogen in the body by far, although circulating levels of estriol are similar to those of other estrogens due to a relatively high rate of metabolism and excretion. Relative to estradiol, both estriol and estrone have far weaker activity as estrogens.

Nandrolone, also known as 19-nortestosterone, is an androgen and anabolic steroid (AAS) which is used in the form of esters such as nandrolone decanoate and nandrolone phenylpropionate. Nandrolone esters are used in the treatment of anemias, cachexia, osteoporosis, breast cancer, and for other indications. They are not used by mouth and instead are given by injection into muscle or fat.
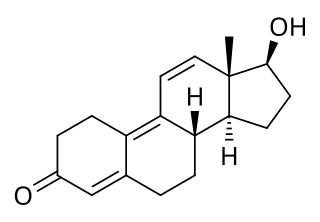
Trenbolone is an androgen and anabolic steroid (AAS) of the nandrolone group which itself was never marketed. Trenbolone ester prodrugs, including trenbolone acetate and trenbolone hexahydrobenzylcarbonate, are or have been marketed for veterinary and clinical use. Trenbolone acetate is used in veterinary medicine in livestock to increase muscle growth and appetite, while trenbolone hexahydrobenzylcarbonate was formerly used clinically in humans but is now no longer marketed. In addition, although it is not approved for clinical or veterinary use, trenbolone enanthate is sometimes sold on the black market under the nickname Trenabol.
Estratetraenol, also known as estra-1,3,5(10),16-tetraen-3-ol, is an endogenous steroid found in women that has been described as having pheromone-like activities in primates, including humans. Estratetraenol is synthesized from androstadienone by aromatase likely in the ovaries, and is related to the estrogen sex hormones, yet has no known estrogenic effects. It was first identified from the urine of pregnant women.

Pregnane, also known as 17β-ethylandrostane or as 10β,13β-dimethyl-17β-ethylgonane, is a C21 steroid and, indirectly, a parent of progesterone. It is a parent hydrocarbon for two series of steroids stemming from 5α-pregnane and 5β-pregnane (17β-ethyletiocholane). It has a gonane core.

Estetrol (E4), or oestetrol, is a weak estrogen steroid hormone, which is found in detectable levels only during pregnancy in humans. It is produced exclusively by the fetal liver. Estetrol is closely related to estriol (E3), which is also a weak estrogen that is found in high quantities only during pregnancy. Along with estradiol (E2), estrone (E1), and E3, estetrol (E4) is a major estrogen in the body, although only during pregnancy.

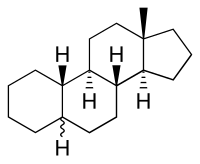
Gonane is a chemical compound with formula C
17H
28, whose molecule can be described as three molecules of cyclohexane and one of cyclopentane, fused in a particular way. More specifically, the molecule can be described as that of cycloheptadecane (–CH2–)17 with three extra bonds connecting carbons 1 to 13, 4 to 12, and 5 to 9, replacing six hydrogen atoms. It can also be viewed as the result of fusing a cyclopentane molecule with a fully hydrogenated molecule of phenanthrene, hence the more descriptive name perhydrocyclopenta[a]phenanthrene.

Moxestrol, sold under the brand name Surestryl, is an estrogen medication which has been used in Europe for the treatment of menopausal symptoms and menstrual disorders. It is taken by mouth. In addition to its use as a medication, moxestrol has been used in scientific research as a radioligand of the estrogen receptor.
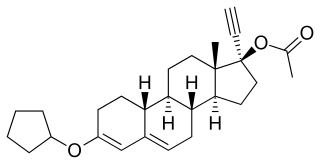
Quingestanol acetate, sold under the brand names Demovis and Pilomin among others, is a progestin medication which was used in birth control pills but is no longer marketed. It is taken by mouth.

Methylestradiol, sold under the brand names Ginecosid, Ginecoside, Mediol, and Renodiol, is an estrogen medication which is used in the treatment of menopausal symptoms. It is formulated in combination with normethandrone, a progestin and androgen/anabolic steroid medication. Methylestradiol is taken by mouth.
Estradiol stearate (E2-17-St), also known as estradiol octadecanoate and sold under the brand name Depofollan, is a naturally occurring estrogen and an estrogen ester – specifically, the C17β stearate ester of estradiol. It occurs in the body as a very long-lasting metabolite and prohormone of estradiol. The compound is one of the components that collectively constitute lipoidal estradiol, another of which is estradiol palmitate. It is extremely lipophilic and hydrophobic. Estradiol stearate has no affinity for the estrogen receptor, requiring transformation into estradiol via esterases for its estrogenic activity. The compound does not bind to sex hormone-binding globulin or α-fetoprotein, instead being transported by lipoproteins such as high-density lipoprotein and low-density lipoprotein.

17α-Epiestriol, or simply 17-epiestriol, also known as 16α-hydroxy-17α-estradiol or estra-1,3,5(10)-triene-3,16α,17α-triol, is a minor and weak endogenous estrogen, and the 17α-epimer of estriol. It is formed from 16α-hydroxyestrone. In contrast to other endogenous estrogens like estradiol, 17α-epiestriol is a selective agonist of the ERβ. It is described as a relatively weak estrogen, which is in accordance with its relatively low affinity for the ERα. 17α-Epiestriol has been found to be approximately 400-fold more potent than estradiol in inhibiting tumor necrosis factor α (TNFα)-induced vascular cell adhesion molecule 1 (VCAM-1) expression in vitro.

Trimethyltrienolone (TMT), also known by its developmental code name R-2956 or RU-2956, is an antiandrogen medication which was never introduced for medical use but has been used in scientific research.

16β,17α-Epiestriol, or 16,17-epiestriol, also known as 16β-hydroxy-17α-estradiol, as well as estra-1,3,5(10)-triene-3,16β,17α-triol, is a minor and weak endogenous steroidal estrogen that is related to 17α-estradiol and estriol. Along with estriol, 16β,17α-epiestriol has been detected in the urine of women during the late pregnancy stage. It shows preferential affinity for the ERβ over the ERα.

Dimethyltrienolone is a synthetic, orally active, and extremely potent anabolic–androgenic steroid (AAS) and 17α-alkylated 19-nortestosterone (nandrolone) derivative which was never marketed for medical use. It has among the highest known affinity of any AAS for the androgen receptors, and has been said to be perhaps the most potent AAS to have ever been developed.

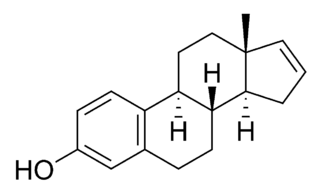
Estrin, or oestrin, also known as estra-1,3,5(10)-triene, is an estrane steroid. It is dehydrogenated estrane with double bonds specifically at the C1, C3, and C5(10) positions. Estrin is a parent structure of the estrogen steroid hormones estradiol, estrone, and estriol, which have also been known as dihydroxyestrin, ketohydroxyestrin, and trihydroxyestrin, respectively.

Ethylestradiol, or 17α-ethylestradiol, also known as 17α-ethylestra-1,3,5(10)-triene-3,17β-diol, is a synthetic estrogen which was never marketed. It occurs as an active metabolite of the anabolic steroids norethandrolone and ethylestrenol formed via aromatase and is believed to be responsible for the estrogenic effects of norethandrolone and ethylestrenol. The 3-methyl ether of ethylestradiol has been used as an intermediate in the synthesis of certain 19-nortestosterone anabolic steroids.

Trendione, also known as estra-4,9,11-triene-3,17-dione, is an androgen prohormone as well as metabolite of the anabolic steroid trenbolone. Trendione is to trenbolone as androstenedione is to testosterone. The compound is inactive itself, showing more than 100-fold lower affinity for the androgen and progesterone receptors than trenbolone. It is a designer steroid and has been sold on the internet as a "nutritional supplement". Trendione is listed in the United States Designer Anabolic Steroid Control Act of 2014.

RU-16117 is an estrogen medication which was investigated for the potential treatment of symptoms of estrogen deficiency such as hot flashes and osteoporosis in women but was never marketed. It was developed for use by mouth.