
Diethyl malonate, also known as DEM, is the diethyl ester of malonic acid. It occurs naturally in grapes and strawberries as a colourless liquid with an apple-like odour, and is used in perfumes. It is also used to synthesize other compounds such as barbiturates, artificial flavourings, vitamin B1, and vitamin B6.

In organic chemistry, nitro compounds are organic compounds that contain one or more nitro functional groups. The nitro group is one of the most common explosophores used globally. The nitro group is also strongly electron-withdrawing. Because of this property, C−H bonds alpha (adjacent) to the nitro group can be acidic. For similar reasons, the presence of nitro groups in aromatic compounds retards electrophilic aromatic substitution but facilitates nucleophilic aromatic substitution. Nitro groups are rarely found in nature. They are almost invariably produced by nitration reactions starting with nitric acid.

Cinnamic acid is an organic compound with the formula C6H5-CH=CH-COOH. It is a white crystalline compound that is slightly soluble in water, and freely soluble in many organic solvents. Classified as an unsaturated carboxylic acid, it occurs naturally in a number of plants. It exists as both a cis and a trans isomer, although the latter is more common.

The Knorr pyrrole synthesis is a widely used chemical reaction that synthesizes substituted pyrroles (3). The method involves the reaction of an α-amino-ketone (1) and a compound containing an electron-withdrawing group α to a carbonyl group (2).
Bromoethane, also known as ethyl bromide, is a chemical compound of the haloalkanes group. It is abbreviated by chemists as EtBr. This volatile compound has an ether-like odor.
The Reformatsky reaction is an organic reaction which condenses aldehydes or ketones with α-halo esters using metallic zinc to form β-hydroxy-esters:
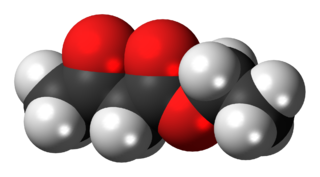
The organic compound ethyl acetoacetate (EAA) is the ethyl ester of acetoacetic acid. It is a colorless liquid. It is widely used as a chemical intermediate in the production of a wide variety of compounds. It is used as a flavoring for food.

Ethyl bromoacetate is the chemical compound with the formula BrCH2CO2CH2CH3. It is the ethyl ester of bromoacetic acid and is prepared in two steps from acetic acid. It is a lachrymator and has a fruity, pungent odor. It is also a highly toxic alkylating agent and may be fatal if inhaled.
2-Chloroethanol (also called ethylene chlorohydrin or glycol chlorohydrin) is an organic chemical compound with the chemical formula HOCH2CH2Cl and the simplest beta-halohydrin (chlorohydrin). This colorless liquid has a pleasant ether-like odor. It is miscible with water. The molecule is bifunctional, consisting of both an alkyl chloride and an alcohol functional group.
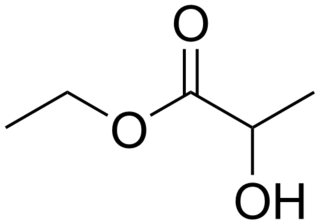
Ethyl lactate, also known as lactic acid ethyl ester, is the organic compound with the formula CH3CH(OH)CO2CH2CH3. It is the ethyl ester of lactic acid. A colorless liquid, it is a chiral ester. Being naturally derived, it is readily available as a single enantiomer. It is commonly used as a solvent. This compound is considered biodegradable and can be used as a water-rinsible degreaser. Ethyl lactate is found naturally in small quantities in a wide variety of foods including wine, chicken, and various fruits. The odor of ethyl lactate when dilute is mild, buttery, creamy, with hints of fruit and coconut.

In organic chemistry, alkyl nitrites are a group of organic compounds based upon the molecular structure R−O−N=O, where R represents an alkyl group. Formally they are alkyl esters of nitrous acid. They are distinct from nitro compounds.

Diethylzinc (C2H5)2Zn, or DEZ, is a highly pyrophoric and reactive organozinc compound consisting of a zinc center bound to two ethyl groups. This colourless liquid is an important reagent in organic chemistry. It is available commercially as a solution in hexanes, heptane, or toluene, or as a pure liquid.

Ethyl chloroformate is an organic compound with the chemical formula ClCO2CH2CH3. It is the ethyl ester of chloroformic acid. It is a colorless, corrosive and highly toxic liquid. It is a reagent used in organic synthesis for the introduction of the ethyl carbamate protecting group and for the formation of carboxylic anhydrides.
2-Methylundecanal is an organic compound that is found naturally in kumquat peel oil. This compound smells herbaceous, orange, and ambergris-like. At high dilution it has a flavor similar to honey and nuts. It is a colorless or pale yellow liquid that is soluble in organic solvents such as ether and ethanol. It is used as a fragrance component in soaps, detergents, and perfumes.

Ethyl iodoacetate is an organic compound with the chemical formula ICH2CO2CH2CH3. It is a derivative of ethyl acetate. Under normal conditions, the compound is a clear, light yellow to orange liquid.

Methyl chloroformate is a chemical compound with the chemical formula Cl−C(=O)−O−CH3. It is the methyl ester of chloroformic acid. It is an oily colorless liquid, although aged samples appear yellow. It is also known for its pungent odor.

Sodium chloroacetate is the organic compound with the formula CH2ClCO2Na. A white, water-soluble solid, it is the sodium salt of chloroacetic acid. Many of its uses are similar to those of the parent acid. It is prepared by treating chloroacetic acid with sodium carbonate.

2-Cyanoacetamide is an organic compound. It is an acetic amide with a nitrile functional group.
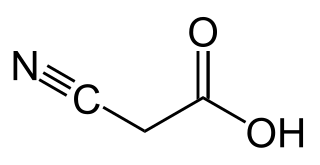
Cyanoacetic acid is an organic compound. It is a white, hygroscopic solid. The compound contains two functional groups, a nitrile (−C≡N) and a carboxylic acid. It is a precursor to cyanoacrylates, components of adhesives.

Ethyl cyanoacetate is an organic compound that contains a carboxylate ester and a nitrile. It is a colourless liquid with a pleasant odor. This material is useful as a starting material for synthesis due to its variety of functional groups and chemical reactivity.


















