
Multiple myeloma (MM), also known as plasma cell myeloma and simply myeloma, is a cancer of plasma cells, a type of white blood cell that normally produces antibodies. Often, no symptoms are noticed initially. As it progresses, bone pain, anemia, kidney dysfunction, and infections may occur. Complications may include amyloidosis.

Lenalidomide, sold under the trade name Revlimid among others, is a medication used to treat multiple myeloma, smoldering myeloma, and myelodysplastic syndromes (MDS). For multiple myeloma, it is used after at least one other treatment and generally with dexamethasone. It is taken by mouth.

Bortezomib, sold under the brand name Velcade among others, is an anti-cancer medication used to treat multiple myeloma and mantle cell lymphoma. This includes multiple myeloma in those who have and have not previously received treatment. It is generally used together with other medications. It is given by injection.

Proteasome inhibitors are drugs that block the action of proteasomes, cellular complexes that break down proteins. They are being studied in the treatment of cancer; and three are approved for use in treating multiple myeloma.

Plasma cell leukemia (PCL) is a plasma cell dyscrasia, i.e. a disease involving the malignant degeneration of a subtype of white blood cells called plasma cells. It is the terminal stage and most aggressive form of these dyscrasias, constituting 2% to 4% of all cases of plasma cell malignancies. PCL may present as primary plasma cell leukemia, i.e. in patients without prior history of a plasma cell dyscrasia or as secondary plasma cell dyscrasia, i.e. in patients previously diagnosed with a history of its predecessor dyscrasia, multiple myeloma. The two forms of PCL appear to be at least partially distinct from each other. In all cases, however, PCL is an extremely serious, life-threatening, and therapeutically challenging disease.

Panobinostat, sold under the brand name Farydak, is a medication used for the treatment of multiple myeloma. It is a hydroxamic acid and acts as a non-selective histone deacetylase inhibitor.

Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare hematologic malignancy. It was initially regarded as a form of lymphocyte-derived cutaneous lymphoma and alternatively named CD4+CD56+ hematodermic tumor, blastic NK cell lymphoma, and agranular CD4+ NK cell leukemia. Later, however, the disease was determined to be a malignancy of plasmacytoid dendritic cells rather than lymphocytes and therefore termed blastic plasmacytoid dendritic cell neoplasm. In 2016, the World Health Organization designated BPDCN to be in its own separate category within the myeloid class of neoplasms. It is estimated that BPDCN constitutes 0.44% of all hematological malignancies.

Carfilzomib, sold under the brand name Kyprolis, is an anti-cancer medication acting as a selective proteasome inhibitor. Chemically, it is a tetrapeptide epoxyketone and an analog of epoxomicin. It was developed by Onyx Pharmaceuticals.
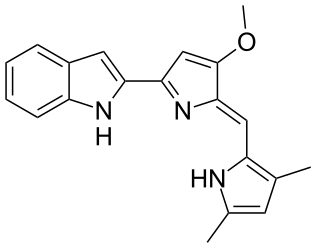
Obatoclax mesylate, also known as GX15-070, is an experimental drug for the treatment of various types of cancer. It was discovered by Gemin X, which was acquired by Cephalon, which has since been acquired by Teva Pharmaceuticals. Several Phase II clinical trials were completed that investigated use of Obatoclax in the treatment of leukemia, lymphoma, myelofibrosis, and mastocytosis.

Ponatinib, sold under the brand name Iclusig, is a medication developed by ARIAD Pharmaceuticals for the treatment of chronic myeloid leukemia (CML) and Philadelphia chromosome–positive (Ph+) acute lymphoblastic leukemia (ALL). It is a multi-targeted tyrosine-kinase inhibitor. Some forms of CML, those that have the T315I mutation, are resistant to current therapies such as imatinib. Ponatinib has been designed to be effective against these types of tumors.

Daratumumab, sold under the brand name Darzalex, is an anti-cancer monoclonal antibody medication. It binds to CD38, which is overexpressed in multiple myeloma cells. Daratumumab was originally developed by Genmab, but it is now being jointly developed by Genmab along with the Johnson & Johnson subsidiary Janssen Biotech, which acquired worldwide commercialization rights to the drug from Genmab.
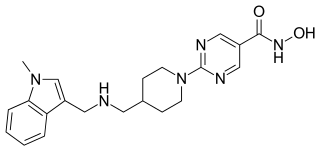
Quisinostat is an experimental drug candidate for the treatment of cancer. It is a "second generation" histone deacetylase inhibitor with antineoplastic activity. It is highly potent against class I and II HDACs.

Immunomodulatory imide drugs (IMiDs) are a class of immunomodulatory drugs containing an imide group. The IMiD class includes thalidomide and its analogues. These drugs may also be referred to as 'Cereblon modulators'. Cereblon (CRBN) is the protein targeted by this class of drugs.

Sonidegib (INN), sold under the brand name Odomzo, is a medication used to treat cancer.

Isatuximab, sold under the brand name Sarclisa, is a monoclonal antibody (mAb) medication for the treatment of multiple myeloma.

Ixazomib is a drug for the treatment of multiple myeloma, a type of white blood cell cancer, in combination with other drugs. It is taken by mouth in the form of capsules.

Melphalan flufenamide, sold under the brand names Pepaxto and Pepaxti, is an anticancer medication used to treat multiple myeloma.

Gedatolisib (PF-05212384) is an experimental drug for treatment of cancer in development by Celcuity, Inc. The mechanism of action is accomplished by binding the different p110 catalytic subunit isoforms of PI3K and the kinase site of mTOR.

Selinexor sold under the brand name Xpovio among others, is a selective inhibitor of nuclear export used as an anti-cancer medication. It works by blocking the action of exportin 1 and thus blocking the transport of several proteins involved in cancer-cell growth from the cell nucleus to the cytoplasm, which ultimately arrests the cell cycle and leads to apoptosis. It is the first drug with this mechanism of action.
Selective inhibitors of nuclear export are drugs that block exportin 1, a protein involved in transport from the cell nucleus to the cytoplasm. This causes cell cycle arrest and cell death by apoptosis. Thus, SINE compounds are of interest as anticancer drugs; several are in development, and one (selinexor) has been approved for treatment of multiple myeloma as a drug of last resort.


















