
The fluorenylmethoxycarbonyl protecting group (Fmoc) is a base-labile protecting group used in organic synthesis.
Contents


The fluorenylmethoxycarbonyl protecting group (Fmoc) is a base-labile protecting group used in organic synthesis.

Fmoc carbamate is frequently used as a protecting group for amines, where the Fmoc group can be introduced by reacting the amine with fluorenylmethyloxycarbonyl chloride (Fmoc-Cl), e.g.: [1]

The other common method for introducing the Fmoc group is through 9-fluorenylmethylsuccinimidyl carbonate (Fmoc-OSu), which may itself be obtained by the reaction of Fmoc-Cl with the dicyclohexylammonium salt of N-hydroxysuccinimide. [2]
Reacting with 9-fluorenylmethyloxycarbonyl azide (itself made by reacting Fmoc-Cl with sodium azide) in sodium bicarbonate and aqueous dioxane is also a method to install Fmoc group. [3]
Because the fluorenyl group is highly fluorescent, certain UV-inactive compounds may be reacted to give the Fmoc derivatives, suitable for analysis by reversed phase HPLC. Analytical uses of Fmoc-Cl that do not use chromatography may be limited by the requirement that excess Fmoc-Cl be removed before an analysis of fluorescence.
The Fmoc group is rapidly removed by base. Piperidine is usually preferred for Fmoc group removal as it forms a stable adduct with the dibenzofulvene byproduct, preventing it from reacting with the substrate. [4] [5]
The use of Fmoc as a temporary protecting group for amine at the N-terminus in SPPS is very widespread for Fmoc/tBu approach, because its removal with piperidine solution does not disturb the acid-labile linker between the peptide and the resin. [6] A typical SPPS Fmoc deprotection is performed with a solution of 20% piperidine in N,N-dimethylformamide (DMF). [7]
Common deprotection cocktails for Fmoc during SPPS:
In chemistry, amines are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines, trimethylamine, and aniline. Inorganic derivatives of ammonia are also called amines, such as monochloramine.

Piperidine is an organic compound with the molecular formula (CH2)5NH. This heterocyclic amine consists of a six-membered ring containing five methylene bridges (–CH2–) and one amine bridge (–NH–). It is a colorless liquid with an odor described as objectionable, typical of amines. The name comes from the genus name Piper, which is the Latin word for pepper. Although piperidine is a common organic compound, it is best known as a representative structure element within many pharmaceuticals and alkaloids, such as natural-occurring solenopsins.

A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis.
In organic chemistry, an acyl chloride is an organic compound with the functional group −C(=O)Cl. Their formula is usually written R−COCl, where R is a side chain. They are reactive derivatives of carboxylic acids. A specific example of an acyl chloride is acetyl chloride, CH3COCl. Acyl chlorides are the most important subset of acyl halides.
Native Chemical Ligation (NCL) is an important extension of the chemical ligation concept for constructing a larger polypeptide chain by the covalent condensation of two or more unprotected peptides segments. Native chemical ligation is the most effective method for synthesizing native or modified proteins of typical size.

In organic chemistry, peptide synthesis is the production of peptides, compounds where multiple amino acids are linked via amide bonds, also known as peptide bonds. Peptides are chemically synthesized by the condensation reaction of the carboxyl group of one amino acid to the amino group of another. Protecting group strategies are usually necessary to prevent undesirable side reactions with the various amino acid side chains. Chemical peptide synthesis most commonly starts at the carboxyl end of the peptide (C-terminus), and proceeds toward the amino-terminus (N-terminus). Protein biosynthesis in living organisms occurs in the opposite direction.
In chemistry, solid-phase synthesis is a method in which molecules are covalently bound on a solid support material and synthesised step-by-step in a single reaction vessel utilising selective protecting group chemistry. Benefits compared with normal synthesis in a liquid state include:
A lactam is a cyclic amide, formally derived from an amino alkanoic acid through cyclization reactions. The term is a portmanteau of the words lactone + amide.

Benzyl chloroformate, also known as benzyl chlorocarbonate or Z-chloride, is the benzyl ester of chloroformic acid. It can be also described as the chloride of the benzyloxycarbonyl group. In its pure form it is a water-sensitive oily colorless liquid, although impure samples usually appear yellow. It possesses a characteristic pungent odor and degrades in contact with water.

The Curtius rearrangement, first defined by Theodor Curtius in 1885, is the thermal decomposition of an acyl azide to an isocyanate with loss of nitrogen gas. The isocyanate then undergoes attack by a variety of nucleophiles such as water, alcohols and amines, to yield a primary amine, carbamate or urea derivative respectively. Several reviews have been published.

Trimethylsilyl chloride, also known as chlorotrimethylsilane is an organosilicon compound, with the formula (CH3)3SiCl, often abbreviated Me3SiCl or TMSCl. It is a colourless volatile liquid that is stable in the absence of water. It is widely used in organic chemistry.

Di-tert-butyl dicarbonate is a reagent widely used in organic synthesis. Since this compound can be regarded formally as the acid anhydride derived from a tert-butoxycarbonyl (Boc) group, it is commonly referred to as Boc anhydride. This pyrocarbonate reacts with amines to give N-tert-butoxycarbonyl or so-called Boc derivatives. These carbamate derivatives do not behave as amines, which allows certain subsequent transformations to occur that would be incompatible with the amine functional group. The Boc group can later be removed from the amine using moderately strong acids. Thus, Boc serves as a protective group, for instance in solid phase peptide synthesis. Boc-protected amines are unreactive to most bases and nucleophiles, allowing for the use of the fluorenylmethyloxycarbonyl group (Fmoc) as an orthogonal protecting group.

The tert-butyloxycarbonyl protecting group or tert-butoxycarbonyl protecting group is a protecting group used in organic synthesis.
Oligonucleotide synthesis is the chemical synthesis of relatively short fragments of nucleic acids with defined chemical structure (sequence). The technique is extremely useful in current laboratory practice because it provides a rapid and inexpensive access to custom-made oligonucleotides of the desired sequence. Whereas enzymes synthesize DNA and RNA only in a 5' to 3' direction, chemical oligonucleotide synthesis does not have this limitation, although it is most often carried out in the opposite, 3' to 5' direction. Currently, the process is implemented as solid-phase synthesis using phosphoramidite method and phosphoramidite building blocks derived from protected 2'-deoxynucleosides, ribonucleosides, or chemically modified nucleosides, e.g. LNA or BNA.

Antamanide is a cyclic decapeptide isolated from a fungus, the death cap: Amanita phalloides. It is being studied as a potential anti-toxin against the effects of phalloidin and for its potential for treating edema. It contains 1 valine residue, 4 proline residues, 1 alanine residue, and 4 phenylalanine residues with a structure of c(Val-Pro-Pro-Ala-Phe-Phe-Pro-Pro-Phe-Phe). It was isolated by determining the source of the anti-phalloidin activity from a lipophillic extraction from the organism. It has been shown that antamanide can react to form alkali metal ion complexes. These include complexes with sodium and calcium ions. When these complexes are formed, the cyclopeptide structure undergoes a conformational change.

Chloroformates are a class of organic compounds with the formula ROC(O)Cl. They are formally esters of chloroformic acid. Most are colorless, volatile liquids that degrade in moist air. A simple example is methyl chloroformate, which is commercially available.

HATU is a reagent used in peptide coupling chemistry to generate an active ester from a carboxylic acid. HATU is used along with Hünig's base, or triethylamine to form amide bonds. Typically DMF is used as solvent, although other polar aprotic solvents can also be used.
Glycopeptides are peptides that contain carbohydrate moieties (glycans) covalently attached to the side chains of the amino acid residues that constitute the peptide.
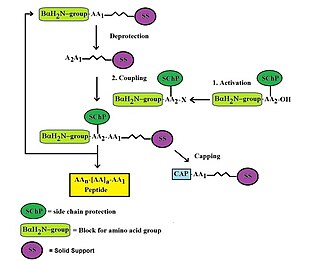
Custom peptide synthesis is the commercial production of peptides for use in biochemistry, biology, biotechnology, pharmacology and molecular medicine. Custom peptide synthesis provides synthetic peptides as valuable tools to biomedical laboratories. Synthetic oligopeptides are used extensively in research for structure-function analysis, for the development of binding assays, the study of receptor agonist/antagonists or as immunogens for the production of specific antibodies. Generally, peptides are synthesized by coupling the carboxyl group or C-terminus of one amino acid to the amino group or N-terminus of another using automated solid phase peptide synthesis chemistries. However, liquid phase synthesis may also be used for specific needs.

DEPBT is a peptide coupling reagent used in peptide synthesis. It shows remarkable resistance to racemization.