
Glass is an amorphous or non-crystalline solid. Because it is often transparent and chemically inert, glass has found widespread practical, technological, and decorative use in window panes, tableware, and optics. Some common objects made of glass like "a glass" of water, "glasses", and a "looking glass", have become named for their material.

Melting, or fusion, is a physical process that results in the phase transition of a substance from a solid to a liquid. This occurs when the internal energy of the solid increases, typically by the application of heat or pressure, which increases the substance's temperature to the melting point. At the melting point, the ordering of ions or molecules in the solid breaks down to a less ordered state, and the solid melts to become a liquid.
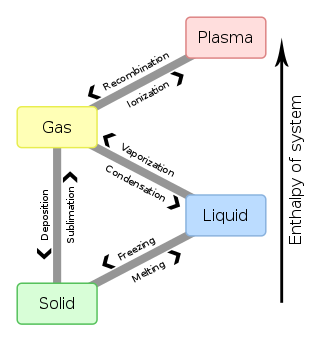
In chemistry, thermodynamics, and other related fields like physics and biology, a phase transition is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic states of matter: solid, liquid, and gas, and in rare cases, plasma. A phase of a thermodynamic system and the states of matter have uniform physical properties. During a phase transition of a given medium, certain properties of the medium change as a result of the change of external conditions, such as temperature or pressure. This can be a discontinuous change; for example, a liquid may become gas upon heating to its boiling point, resulting in an abrupt change in volume. The identification of the external conditions at which a transformation occurs defines the phase transition point.

Supercooling, also known as undercooling, is the process of lowering the temperature of a liquid below its freezing point without it becoming a solid. It is achieved in the absence of a seed crystal or nucleus around which a crystal structure can form. The supercooling of water can be achieved without any special techniques other than chemical demineralization, down to −48.3 °C (−54.9 °F). Supercooled water can occur naturally, for example in the atmosphere, animals or plants.
Vitrification is the full or partial transformation of a substance into a glass, that is to say, a non-crystalline amorphous solid. Glasses differ from liquids structurally and glasses possess a higher degree of connectivity with the same Hausdorff dimensionality of bonds as crystals: dimH = 3. In the production of ceramics, vitrification is responsible for their impermeability to water.

An amorphous metal is a solid metallic material, usually an alloy, with disordered atomic-scale structure. Most metals are crystalline in their solid state, which means they have a highly ordered arrangement of atoms. Amorphous metals are non-crystalline, and have a glass-like structure. But unlike common glasses, such as window glass, which are typically electrical insulators, amorphous metals have good electrical conductivity and can show metallic luster.
In materials science and continuum mechanics, viscoelasticity is the property of materials that exhibit both viscous and elastic characteristics when undergoing deformation. Viscous materials, like water, resist shear flow and strain linearly with time when a stress is applied. Elastic materials strain when stretched and immediately return to their original state once the stress is removed.

Polyamorphism is the ability of a substance to exist in several different amorphous modifications. It is analogous to the polymorphism of crystalline materials. Many amorphous substances can exist with different amorphous characteristics. However, polyamorphism requires two distinct amorphous states with a clear, discontinuous (first-order) phase transition between them. When such a transition occurs between two stable liquid states, a polyamorphic transition may also be referred to as a liquid–liquid phase transition.

Aerodynamic levitation is the use of gas pressure to levitate materials so that they are no longer in physical contact with any container. In scientific experiments this removes contamination and nucleation issues associated with physical contact with a container.
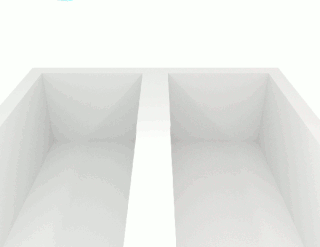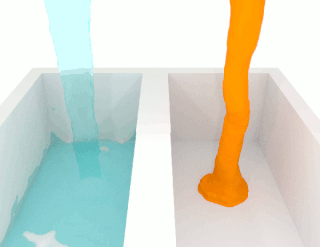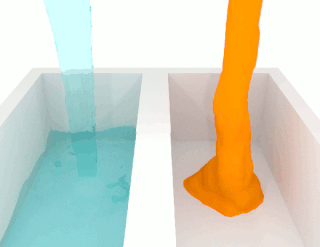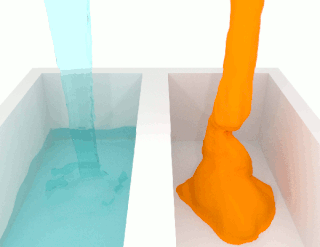
The viscosity of a fluid is a measure of its resistance to deformation at a given rate. For liquids, it corresponds to the informal concept of "thickness": for example, syrup has a higher viscosity than water. Viscosity is defined scientifically as a force multiplied by a time divided by an area. Thus its SI units are newton-seconds per square meter, or pascal-seconds.

The glass–liquid transition, or glass transition, is the gradual and reversible transition in amorphous materials from a hard and relatively brittle "glassy" state into a viscous or rubbery state as the temperature is increased. An amorphous solid that exhibits a glass transition is called a glass. The reverse transition, achieved by supercooling a viscous liquid into the glass state, is called vitrification.
Johari–Goldstein relaxation, also known as the JG β-relaxation, is a universal property of glasses and certain other disordered materials. Proposed in 1969 by Martin Goldstein, JG β-relaxation were described as a secondary relaxation mechanism required to explain the viscosity behavior of liquids approaching the glass transition in the potential energy landscape picture presented in Goldstein's seminal 1969 paper. Previous experiments on glass forming liquids showed multiple relaxation times present in liquids measured by time dependent compliance measurements. Gyan Johari and Martin Goldstein measured the dielectric loss spectrum of a set of rigid glass forming molecules to further test the hypothesis of Goldstein in 1969. The relaxation, a peak in mechanical or dielectric loss at a particular frequency, had previously been attributed to a type of molecular flexibility. The fact that such a loss peak shows up in glasses of rigid molecules lacking this flexibility demonstrated its universal character.
In condensed matter physics and physical chemistry, the terms viscous liquid, supercooled liquid, and glass forming liquid are often used interchangeably to designate liquids that are at the same time highly viscous, can be or are supercooled, and able to form a glass.
Charles Austen Angell was a renowned Australian and American physical chemist known for his prolific and highly cited research on the chemistry and physics of glasses and glass-forming liquids. He was internationally recognized as a luminary in the fields of glasses, liquids, water and ionic liquids.
In polymer chemistry and polymer physics, the Flory–Fox equation is a simple empirical formula that relates molecular weight to the glass transition temperature of a polymer system. The equation was first proposed in 1950 by Paul J. Flory and Thomas G. Fox while at Cornell University. Their work on the subject overturned the previously held theory that the glass transition temperature was the temperature at which viscosity reached a maximum. Instead, they demonstrated that the glass transition temperature is the temperature at which the free space available for molecular motions achieved a minimum value. While its accuracy is usually limited to samples of narrow range molecular weight distributions, it serves as a good starting point for more complex structure-property relationships.
Dynamical heterogeneity describes the behavior of glass-forming materials when undergoing a phase transition from the liquid state to the glassy state. In dynamical heterogeneity, the dynamics of cooling to a glassy state show variation within the material.
Vitrimers are a class of plastics, which are derived from thermosetting polymers (thermosets) and are very similar to them. Vitrimers consist of molecular, covalent networks, which can change their topology by thermally activated bond-exchange reactions. At high temperatures they can flow like viscoelastic liquids, at low temperatures the bond-exchange reactions are immeasurably slow (frozen) and the Vitrimers behave like classical thermosets at this point. Vitrimers are strong glass formers. Their behavior opens new possibilities in the application of thermosets like as a self-healing material or simple processibility in a wide temperature range.
Alessio Zaccone is an Italian physicist.
The Vogel–Fulcher–Tammann equation, also known as Vogel–Fulcher–Tammann–Hesse equation or Vogel–Fulcher equation, is used to describe the viscosity of liquids as a function of temperature, and especially its strongly temperature dependent variation in the supercooled regime, upon approaching the glass transition. In this regime the viscosity of certain liquids can increase by up to 13 orders of magnitude within a relatively narrow temperature interval.











