Related Research Articles
A monomer is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization.

A polymer is a substance or material consisting of very large molecules, or macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compounds, produces unique physical properties including toughness, high elasticity, viscoelasticity, and a tendency to form amorphous and semicrystalline structures rather than crystals.

Natural rubber, also called India rubber, latex, Amazonian rubber, caucho or caoutchouc, as initially produced, consists of polymers of the organic compound isoprene, with minor impurities of other organic compounds, plus water. Thailand and Indonesia are two of the leading rubber producers. Types of polyisoprene that are used as natural rubbers are classified as elastomers.

Vulcanization refers to a range of processes for hardening rubbers. The term originally referred exclusively to the treatment of natural rubber with sulfur, which remains the most common practice; however, it has also grown to include the hardening of other (synthetic) rubbers via various means. Examples include silicone rubber via room temperature vulcanizing and chloroprene rubber (neoprene) using metal oxides.

Gutta-percha is a tree of the genus Palaquium in the family Sapotaceae. The name also refers to the rigid, naturally biologically inert, resilient, electrically nonconductive, thermoplastic latex produced from the sap of the tree, particularly from Palaquium gutta; it is a polymer of isoprene which forms a rubber-like elastomer.
Polymer chemistry is a sub-discipline of chemistry that focuses on the chemical synthesis, structure, and chemical and physical properties of polymers and macromolecules. The principles and methods used within polymer chemistry are also applicable through a wide range of other chemistry sub-disciplines like organic chemistry, analytical chemistry, and physical chemistry. Many materials have polymeric structures, from fully inorganic metals and ceramics to DNA and other biological molecules, however, polymer chemistry is typically referred to in the context of synthetic, organic compositions. Synthetic polymers are ubiquitous in commercial materials and products in everyday use, commonly referred to as plastics, and rubbers, and are major components of composite materials. Polymer chemistry can also be included in the broader fields of polymer science or even nanotechnology, both of which can be described as encompassing polymer physics and polymer engineering.

Styrene-butadiene or styrene-butadiene rubber (SBR) describe families of synthetic rubbers derived from styrene and butadiene. These materials have good abrasion resistance and good aging stability when protected by additives. In 2012, more than 5.4 million tonnes of SBR were processed worldwide. About 50% of car tires are made from various types of SBR. The styrene/butadiene ratio influences the properties of the polymer: with high styrene content, the rubbers are harder and less rubbery. SBR is not to be confused with the thermoplastic elastomer, styrene-butadiene block copolymer, although being derived from the same monomers.
Viton is a brand of FKM, a synthetic rubber and fluoropolymer elastomer commonly used in seals, chemical-resistant gloves, and other molded or extruded goods. The name is a registered trademark of The Chemours Company previous Du Pont de Nemours and was introduced in 1957.

An elastomer is a polymer with viscoelasticity and has very weak intermolecular forces, generally low Young's modulus and high failure strain compared with other materials. The term, a portmanteau of elastic polymer, is often used interchangeably with rubber, although the latter is preferred when referring to vulcanisates. Each of the monomers which link to form the polymer is usually a compound of several elements among carbon, hydrogen, oxygen and silicon. Elastomers are amorphous polymers maintained above their glass transition temperature, so that considerable molecular reconformation, without breaking of covalent bonds, is feasible. At ambient temperatures, such rubbers are thus relatively compliant and deformable. Their primary uses are for seals, adhesives and molded flexible parts. Application areas for different types of rubber are manifold and cover segments as diverse as tires, soles for shoes, and damping and insulating elements. The importance of these rubbers can be judged from the fact that global revenues are forecast to rise to US$56 billion in 2020.

Polysulfides are a class of chemical compounds containing chains of sulfur atoms. There are two main classes of polysulfides: anions and organic polysulfides. Anions have the general formula S2−
n. These anions are the conjugate bases of the hydrogen polysulfides H2Sn. Organic polysulfides generally have the formulae RSnR, where R = alkyl or aryl.
A synthetic rubber is any artificial elastomer. They are polymers synthesized from petroleum byproducts. About 32-million metric tons of rubbers are produced annually in the United States, and of that amount two thirds are synthetic. Global revenues generated with synthetic rubbers are likely to rise to approximately US$56 billion in 2020. Synthetic rubber, just like natural rubber, has many uses in the automotive industry for tires, door and window profiles, seals such as O-rings and gaskets, hoses, belts, matting, and flooring. They offer a different range of physical and chemical properties, so can improve the reliability of a given product or application. Synthetic rubbers are superior to natural rubbers in two major respects, thermal stability and resistance to oils and related compounds. They are more resistant to oxidizing agents for example, such as oxygen and ozone which can reduce the life of products like tyres.
Anionic addition polymerization is a form of chain-growth polymerization or addition polymerization that involves the polymerization of monomers initiated with anions. The type of reaction has many manifestations, but traditionally vinyl monomers are used. Often anionic polymerization involves living polymerizations, which allows control of structure and composition.

Polybutadiene [butadiene rubber BR] is a synthetic rubber. Polybutadiene rubber is a polymer formed from the polymerization of the monomer 1,3-butadiene. Polybutadiene has a high resistance to wear and is used especially in the manufacture of tires, which consumes about 70% of the production. Another 25% is used as an additive to improve the toughness of plastics such as polystyrene and acrylonitrile butadiene styrene (ABS). Polybutadiene rubber accounted for about a quarter of total global consumption of synthetic rubbers in 2012. It is also used to manufacture golf balls, various elastic objects and to coat or encapsulate electronic assemblies, offering high electrical resistivity.

Polymer science or macromolecular science is a subfield of materials science concerned with polymers, primarily synthetic polymers such as plastics and elastomers. The field of polymer science includes researchers in multiple disciplines including chemistry, physics, and engineering.
A polyolefin is a type of polymer produced from a simple olefin as a monomer. For example, polyethylene is the polyolefin produced by polymerizing the olefin ethylene. Polypropylene is another common polyolefin which is made from the olefin propylene.
Nitrile rubber, also known as NBR, Buna-N, and acrylonitrile butadiene rubber, is a synthetic rubber derived from acrylonitrile (ACN) and butadiene. Trade names include Perbunan, Nipol, Krynac and Europrene. This rubber is unusual in being resistant to oil, fuel, and other chemicals.
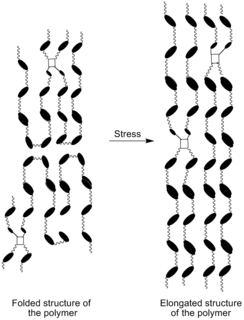
Strain crystallization is a phenomenon in which an initially amorphous solid material undergoes a phase transformation due to the application of strain. Strain crystallization occurs in natural rubber, as well as other elastomers and polymers. The phenomenon has important effects on strength and fatigue properties.

The Charles Goodyear Medal is the highest honor conferred by the American Chemical Society, Rubber Division. Established in 1941, the award is named after Charles Goodyear, the discoverer of vulcanization, and consists of a gold medal, a framed certificate and prize money. The medal honors individuals for "outstanding invention, innovation, or development which has resulted in a significant change or contribution to the nature of the rubber industry". Awardees give a lecture at an ACS Rubber Division meeting, and publish a review of their work in the society's scientific journal Rubber Chemistry and Technology.
Samuel Emmett Horne Jr. was a research scientist at B. F. Goodrich noted for first synthesizing cis-1,4-polyisoprene, the main polymer contained in natural tree rubber, using Ziegler catalysis. Earlier attempts to produce synthetic rubber from isoprene had been unsuccessful, but in 1955, Horne prepared 98 percent cis-1,4-polyisoprene via the stereospecific polymerization of isoprene. The product of this reaction differs from natural rubber only slightly. It contains a small amount of cis-1,2-polyisoprene, but it is indistinguishable from natural rubber in its physical properties.
Synthetic biopolymers are human-made copies of biopolymers obtained by abiotic chemical routes. Synthetic biopolymer of different chemical nature have been obtained, including polysaccharides, glycoproteins, peptides and proteins, polyhydroxoalkanoates, polyisoprenes.
References
- ↑ "Audio interview with FW Stavely".
- ↑ Morris, Peter J. T. (2005). Polymer Pioneers: A Popular History of the Science and Technology of Large Molecules. Chemical Heritage Foundation. p. 88. ISBN 9780941901031.
| This biographical article about an American chemist is a stub. You can help Wikipedia by expanding it. |